
Chemotherapeutics in the Treatment of Periodontal Diseases
Treatment strategies combining the use of mechanical therapies and adjunctive chemotherapeutics enable clinicians to better manage periodontal diseases.
Oral healthcare professionals continually seek ways to address the plaque biofilm and excessive host response that are responsible for the breakdown of connective tissue and bone associated with periodontal diseases. While mechanical therapy (such as scaling, root planing and power instrumentation) is considered the gold standard for biofilm disruption and removal, the data tell us that clinicians rarely if ever achieve complete removal of the putative pathogens that reside in the biofilm from the surface.1 What scientists do know is that the plaque biofilm is composed of a complex group of bacteria living together in a multispecies community. The microbes in the biofilm are stable, and tightly adhere to each other and to an oral substrate by means of an extracellular matrix.2,3 Bacteria in biofilms are much more resistant to antimicrobial agents than those dispersed as single cells of the same species.
 Knowing this helps us to understand why antibiotics utilized to address the biofilm, either locally applied or systemically administered, are considered adjuncts to mechanical therapy instead of monotherapies. What this means to dental clinicians is that an optimal therapeutic approach may include adjunctive therapies such as antiseptics and/or antibiotics, as well as host modulation (in addition to mechanical therapy) to best address a complex disease process. Devising a suitable treatment plan would also depend, of course, on the patient’s host response and oral healthcare habits.
Knowing this helps us to understand why antibiotics utilized to address the biofilm, either locally applied or systemically administered, are considered adjuncts to mechanical therapy instead of monotherapies. What this means to dental clinicians is that an optimal therapeutic approach may include adjunctive therapies such as antiseptics and/or antibiotics, as well as host modulation (in addition to mechanical therapy) to best address a complex disease process. Devising a suitable treatment plan would also depend, of course, on the patient’s host response and oral healthcare habits.
Chemotherapeutic agents are used to eliminate, reduce or alter the effect of microorganisms in the oral cavity and elevated levels of pro-inflammatory mediators. The term antimicrobial refers to agents that kill microbes or affect the growth and multiplication of microorganisms.4 Several chemotherapeutic agents are available to oral healthcare providers to assist in the control and reduction of supragingival plaque and associated gingivitis. These typically take the form of mouth rinses or dentifrices. Other agents available for the control and treatment of chronic periodontitis, such as locally applied antimicrobials/antibiotics and systemically administered antimicrobials, are reserved for more aggressive cases.
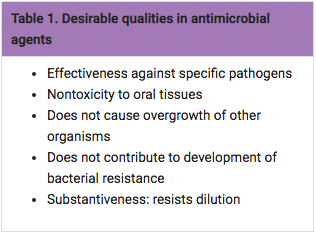
As noted in Table 1, a valuable quality in antimicrobial agents is substantiveness, or the ability of an agent to remain in an area or site and resist being diluted or washed away by gingival crevicular fluid or salivary action.5 In addition, a systemically administered sub-antimicrobial dose of doxycycline (20 mg) has been approved by the United States Food and Drug Administration (FDA) as an adjunctive host modulatory therapy to reduce levels of enzymes and cytokines known to drive the breakdown of connective tissue and bone metabolism changes associated with periodontitis.
Mouthrinses and Dentrifices
Mouthrinses designed to reduce plaque and gingivitis contain antiseptic agents—chemical antimicrobial agents that are applied topically or subgingivally to mucous membranes, wounds or intact dermal surfaces to destroy microorganisms, inhibit their reproduction or inhibit their metabolism. While most antiseptics are bactericidal (meaning, kills bacteria), some are bacteriostatic (that is, they inhibit growth and reproduction of bacteria without killing them). The most common active ingredients in mouthrinses are essential oils of dentifrice: chlorhexidine gluconate (CHX—available by prescription only); cetylpyridinium chloride (CPC) and stannous fluoride. These agents have been evaluated in the laboratory (pre-clinically) and in clinical trials for efficacy and safety. All are safe when used as directed. Chemotherapeutic dentifrices in the U.S. primarily contain triclosan or stannous fluoride.
Here is a brief description of the active ingredients in common chemotherapeutic mouthrinses and dentifrices used in the control of plaque and gingivitis:
Chlorhexidine(CHX): The antiseptic CHX is one of the most effective antiplaque/antigingivitis agents available to clinicians. It is sold in the U.S. as a prescription mouthrinse at 0.12 percent concentration. Most products contain 11.6 percent alcohol, although products without alcohol are also available. The mechanism of action is related to an alteration of bacterial adsorption, a reduction in pellicle formation and an alteration of the bacterial cell wall causing lysis to occur. A major advantage of CHX is that it is a very substantive agent that brings reductions in plaque biofilm and gingivitis ranging from 22 to 61% and 18 to 44%, respectively.6 The disadvantages of CHX are teeth staining, alteration of taste and an increase in calcified deposits.
Essential Oils: The three essential oils are a mixture of thymol, menthol and eucalyptol combined with methyl salicylate. The alcohol content is 21.0 to 26.9%. The mechanism of action is related to alteration of the bacterial cell wall. Studies have reported plaque and gingivitis reductions ranging from 14 to 56% and 14 to 39%, respectively.6 The adverse effect is a burning sensation during use with certain formulations.
Cetylpyridinium Chloride (CPC): CPC is classified as a quaternary ammonium compound. The mechanisms of action include: rupture of cell walls; the promotion of cell lysis; decreased cell metabolism; and the ability for bacteria to attach to tooth surfaces. Studies have reported plaque and gingivitis reductions of 15% and 24%, respectively.7,8 Reported side effects include staining, increased calculus formation and occasional burning.
Stannous Fluoride (SnF2): SnF2 products are available in rinses and dentifrices. Formulations vary from 0.63% in rinses to 0.045% in dentifrices. Previous reports showed a reduction in plaque biofilm and gingivitis for short periods after use. A possible downside is that formulations have received mixed reviews in the past due to the instability of stannous fluoride (although new, more stable formulations are available). Reported side effects include staining and altered taste.
Triclosan: This is a broad spectrum antibacterial agent. In the U.S., triclosan (0.03%) is only available in a dentifrice with the copolymer Gantrez (2.0%), which improves its efficacy. With the addition of 0.243 sodium fluoride in a silica base, it has demonstrated efficacy in reducing plaque, gingivitis calculus and dental caries.9 Reductions in plaque and gingivitis ranging from 12 to 59% and 22 to 25%, respectively, have been reported.10 Triclosan has been used safely for many years.
Chemotherapeutic Agents for Chronic Periodontitis

Although many patients will respond to thorough debridement and self-care therapies such as described above, for patients who do not improve or for whom their periodontal health continues to decline, stronger adjunctive therapies may be necessary. Since the 1980s, locally applied antimicrobials/ antibiotics (LAAs) have been available to dental professionals. (See Table 2.)
Currently, three resorbable, site-specific locally administered antimicrobial/antibiotics products approved by the FDA for the treatment of chronic periodontitis are available. They are: Arestin® (OraPharma, Inc. Warminster, Penn.), minocycline microspheres; Atridoxâ„¢ (Tolmar Inc. Ft. Collins, Colo.), a doxycycline gel; and Perio Chip® (Dexcel Pharma, Alzenau, Germany), a chlorhexidine based chip. PerioChip is the only antiseptic LAA; it is not an antibiotic. Patients who have an allergy to the tetracycline class of drugs or who are pregnant should have Perio Chip placed if an LAA is indicated.
These agents, used as an adjunct to scaling and root planing, deliver an antiseptic or antibiotic to the base of the periodontal pocket with the goal of improving pocket depth reductions, clinical attachment level gains and reductions in bleeding on probing. The substantivity of the agents vary (seven days for chlorhexidine glucontate11 and doxycycline;12 14 to 21 days for minocycline13). However, all have greater substantivity than mouthrinses used subgingivally as irrigants. The added benefit of the agents is the slow release of the active ingredients at a higher minimal inhibitory concentration (MIC) level than can be achieved by any other application. The MIC represents the concentration of antibiotic required to inhibit growth of a planktonic bacterial population. Locally delivered, site-specific agents have a higher and longer substantivity and maintain MIC long enough to significantly reduce the level of pathogens, leading to improvements in the periodontal condition after single or multiple applications.
While all of the agents were studied in nine-month clinical trials for the improvement of chronic periodontitis, more recent investigations continue to show benefits for highrisk patients. For example, Goodson et al demonstrated that Arestinâ„¢ significantly reduces periodontal pathogens comprising the “red complex” (i.e., P gingivalis, T. forsythia and T. denticola), compared to scaling and root planing alone by one month, particularly in smokers.14 A two-year follow-up of Atridoxâ„¢ and scaling and root planing in smokers demonstrated sustained improvements in probing depth reductions and relative attachment level gains beyond what was achieved with scaling and root planing alone.15 These findings are important for clinicians who strive to improve the periodontal condition of patients who do not respond to mechanical therapy. Recent studies have demonstrated the intensive periodontal therapy with locally applied antimicrobials also results in significant reduction in the overall inflammatory burden, with reduced risk for cardiovascular events.16,17
Other Locally Applied Products
 Periowave (Periowave Dental Technologies, Inc. Toronto, Ontario, Canada), currently not approved for use in the U.S., is a non-antibiotic therapy intended to destroy gram negative bacteria without promoting the development of bacterial resistance.18 Used in the treatment of chronic periodontitis, it utilizes a cold (non-thermal), low-power diode laser as the activating light. Indications for use are for patients with 4 to 9 mm pockets that bleed on probing.
Periowave (Periowave Dental Technologies, Inc. Toronto, Ontario, Canada), currently not approved for use in the U.S., is a non-antibiotic therapy intended to destroy gram negative bacteria without promoting the development of bacterial resistance.18 Used in the treatment of chronic periodontitis, it utilizes a cold (non-thermal), low-power diode laser as the activating light. Indications for use are for patients with 4 to 9 mm pockets that bleed on probing.
Marketed as a method to treat chronic periodontitis, Perio Protect® (Perio Protect LLC, St. Louis, Mo.) relies on a custom-made tray to deliver a solution (selected by the dentist) to chemically alter the biofilm in the periodontal pocket and change the pocket’s microbiological environment to disrupt biofilm growth. In November 2009, the American Academy of Periodontology (AAP) published a fact sheet on its consumer website to educate the public about Perio Protect.19 The AAP reported that it was not aware of any randomized, controlled clinical trials published in peer-reviewed scientific journals on the efficacy of this therapy. FDA approval relative to this product is for the tray as a device only.
In the 1980s it was recognized that although bacteria may initiate the periodontal disease process, they were insufficient by themselves to cause peridontitis. Research supported the fact that a person’s response to the perio dontal pathogens, known as the host response, was key to the development and progression of the disease. Elevations in pro-inflammatory mediators (such as cytokines, prostanoids and enzymes known as matrix lo proteinases) were driving the disease process, leading to the connective tissue breakdown and bone metabolism changes pathognomonic of periodontitis. These findings resulted in thedev elopment of an FDA-approved host modulatory therapy known as Periostat. This therapy evolved as a new use for an old drug, doxycycline, which when used at a sub-antimicrobial dose (20mg) could inhibit the destructive enzymes and reduce the excessive levels of cytokines associated with active disease.
When administered at this sub-antimicrobial dose, doxycycline does not cause the long-term side effects seen with high doses of antibiotics such as gastrointestinal upset, the overgrowth of yeast and the development of bacterial resistance. Periostat was tested in multiple randomized double blind placebo controlled clinical trials and found to be an effective adjunct to scaling and root planing, contributing to significant improvements in probing depth reductions, clinical attachment level gains, and reductions in bleeding on probing with no adverse effects.20 It has been shown to boost the effects of locally applied antimicrobials such as Atridox.21 Recent clinical studies have supported its use in high-risk patients who are more difficult to manage, such as smokers,22 people with diabetes23 and women with osteoporosis/ osteo penia,24 as well as those at risk for cardiovascular disease.25 It is available in a generic form by prescription only and should be used for a minimum of three months.
Conclusion
Dental professionals have come a long way in developing strategies that help in the prevention and treatment of periodontitis. Treatment strategies combining the use of mechanical therapies and adjunctive chemotherapeutics enable us to better manage both gingivitis and periodontitis. The use of antiseptics, antibiotics and host modulatory therapy as adjuncts to brushing, ultrasonics, scaling and root planing have made non-surgical therapies more predictable, resulting in improvements in plaque control, pocket depth reductions, clinical attachment levels and bleeding.
The implementation of intensive periodontal therapy may not only improve the oral condition of patients, but may also have a positive impact on their overall health. Re-evaluation and constant monitoring of patients is essential when managing a chronic progressive disease, and clinicians must recognize that when nonsurgical therapy is not enough, they must consider surgical approaches to manage periodontal diseases. We are fortunate to be practicing in an era where we have the tools to treat a wide array of patients with varying risk for the development and progression of peridontitis. It is up to us to plan treatment strategies that address the unique needs of each patient.
References
- Kepic TJ, O’Leary TJ, Kafrawy AH. Total calculus removal: an attainable objective? Periodontol. 1990; 61:16-20.
- Listgarten MA. Structure of the microbial flora associated with periodontal health and diseases in man. J Periodontol. 1976;47:1-18.
- Cobb CM, Killoy WJ. Microbial colonization in human periodontal disease: an illustrated tutorial on selected ultrastructural and ecologic considerations. Scan Microsc. 1990;4:675-691.
- American Academy of Periodontology: AAP guidelines for periodontal therapy. J Periodontal. 2001;72:1624-1628.
- Elworthy A, Greenman J, Doherty FM et al. The substantivity of a number of oral hygiene products determined by the duration of effects on salivary bacteria. J Peroidontal. 1996;76;572-576.
- Addy M. Chlorhexidine compared with other locally delivered antimicrobials. J Clin Periodontol. 1986;13:957-964.
- Lobene RR, Lobene S, Soparkar PM. The effect of a cetylpyridinium chloride mouthwash on plaque and gingivitis. J Dent Res. 1977;56:595.
- Allen DR, Davies R, Bradshaw B et al. Efficacy of a mouthrinse containing 0.05% cetylpyridinium chloride for the control of plaque and gingivitis: a six-month study in adults. Compend Contin Educ Dent. 1998;19:20-26.
- Volpe AR, Petrone ME, DeVizio W et al. A review of plaque, gingivitis, calculus and caries clinical efficacy with a dentifrice containing triclosan and PVM/MA Copolymer. J Clin Dent. 1993;4:31-41.
- Cubells AB, Dalmau LB, Petrone ME et al. The effect of a triclosan/copolymer/fluoride dentifrice on plaque formation and gingivitis: a six-month study. J Clint Dent. 1991;2:63-69.
- Jeffcoat MK, Bray KS, Ciancio SG et al. Adjunctive use of a a subgingival controlled-release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing along. J Periodontol. 1998;69:989-997.
- Stoller NH, Johnson LR, Trapnell S et al. The pharmacokinetic profile of a biodegradable controlled release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva, and serum. J Periodontol. 1998;69:1085-91.
- Christersson LA. Tissue response and release of minocycline after subgingival deposition by use of a resorbable polymer. Warminster, Pa: OraPharma Inc; 1988.
- Goodson JM, Gunsolley JC, Grossi SG et al. Minocycline HCI microspheres reduce red-complex bacteria in periodontal disease therapy. J Periodontol. 2007;78:1568-79.
- Machion L, Andia DC, Lecio G et al. Locally delivered doxycycline as an adjunctive therapy to scaling and root planing in the treatment of smokers: a two-year follow-up. J Periodontol. 2006 Apr;77(4):606-13.
- D’Aiuto F, Parkar M, Nibali L et al. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Heart J. 2006 May; 151(5):977-84.
- Tonetti MS, D’Aiuto F, Nibali L et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007 Mar 1;356(9):911-20.
- http://www.periowave.com. Accessed April 16, 2010.
- http://www.perioprotect.com/whatIs.asp. Accessed April 16, 2010.
- Ciancio S, Ashley R. Safety and efficacy of sub-antimicrobial-dose doxycycline therapy in patients with adult periodontitis. Adv Dent Res. 1998 Nov;12(2):27-31.
- Novak MJ, Dawson DR 3rd, Magnusson I et al. Combining host modulation and topical antimicrobial therapy in the management of moderate to severe periodontitis: a randomized multicenter trial. J Periodontol. 2008 Jan;79(1):33-41.
- Preshaw PM, Hefti AF, Bradshaw MH et al. Adjunctive subantimicrobial dose doxycycline in smokers and non-smokers with chronic periodontitis. J Clin Periodontol. 2005 Jun;32(6):610-6.
- Martorelli de Lima AF, Cury CC et al. Therapy with adjunctive doxycycline local delivery in patients with type 1 diabetes mellitus and periodontitis. J Clin Periodonol. 2004 Aug;31(8):648-53.
- Reinhardt RA, Stoner JA, Golub LM et al. Efficacy of sub-antimicrobial dose doxycycline in post menopausal women: clinicaloutcomes. J Clin Periodontol. 2007 Sep;34(9):768-75.
- Brown DL, Desai KK, Vakili BA. Et al. Clinical and Biochemical Results of the Metalloproteinase Inhibition with Subantimicrobial Doses of Doxycycline to Prevent Acute Coronary Syndromes (MIDAS) Pilot Trial. Arterioscler Thromb Vasc Biol. 2004;24:733-738.
From Dimensions of Dental Hygiene. June 2010; 8(6): 44-46, 48.

