 PIOTR MARCINSKI / ISTOCK / THINKSTOCK
PIOTR MARCINSKI / ISTOCK / THINKSTOCK
Chairside Management of Dentinal Hypersensitivity
In-office treatment of this common dental complaint can provide effective relief.
Dentinal hypersensitivity (DH) remains a common condition affecting 10% to 30% of adults.1 The pain produced by DH can range from subtle—serving as a minor annoyance—to severe, in which it disrupts daily activities. DH can significantly impact quality of life, including the ability to comfortably perform routine oral hygiene. Patients may change their habits to prevent sensitivity by avoiding hot or cold foods/beverages and/or eliminating offending items from their diets.
This article will focus on the chairside treatment of DH, but at-home strategies are also integral to sensitivity management. Dental professionals should be able to provide recommendations on toothpastes, mouthrinses, and pastes to help patients initially address DH at home.2 These strategies are minimally invasive and treat general regions (multiple teeth/arch) of exposed cervical dentin, nonspecifically.

OVERSEAS / COLLECTION CNRI / SPL
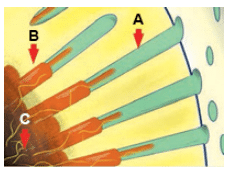
Clinical treatments of DH are based on the hydrodynamic theory, a well-accepted understanding of sensitivity’s mechanism of action.3 Pain results from fluids within exposed dentinal tubules becoming disturbed either by temperature, physical, or osmotic changes. These fluid movements stimulate a baroreceptor that produces a neural signal. For example, when a thermal stimulus is applied to a cervical site with patent dentinal tubules, it causes fluid movement within the tubules—resulting in depolarization of nociceptors (pain receptors). Similarly, applied air to a site will desiccate the surface, prompting peripheral flow of fluid toward the dehydrated surface (Figure 2). This fluid movement also depolarizes the nociceptors that evoke the same response. Consequently, the treatment of DH—whether in office or at home—focuses on two goals: occluding the dentinal tubules and otherwise impeding the stimulation of pulpal nociceptors. A variety of clinical approaches to in-office DH treatment are available.
FLUORIDE
In-office fluoride applications include gels or varnishes applied to the affected areas. These produce a partial or complete blockage of dentinal tubules by forming calcium fluoride (CaF2) or calcium-phosphate precipitates. In dentifrices, fluoride is found in combination with other tubule-obstructing agents that contain potassium or oxalates. One randomized control study found two chairside varnishes to be equally effective in reducing dentinal symptomology when compared with baseline.4 These 5% sodium fluoride varnishes produced sustained improvements for up to 6 months. A fluoride gel (1.23% NaF) and stannous fluoride (SnF2 0.63% in an aqueous solution) also showed promise.5,6 Many products are designed for convenient, single-dose application. Other products containing topical fluorides may also be useful.
GLUTARALDEHYDE/HYDROXYETHYL METHACRYLATE
Products containing 5% glutaraldehyde and 35% hydroxyethyl methacrylate (HEMA) are also used to treat DH. Studies show that the use of these products can alleviate symptoms by 5% to 27% for 7 months to 9 months.7 Glutaraldehyde/HEMA reacts with serum albumin proteins in dentinal fluid to form a precipitate that narrows or fully blocks dentinal tubules. Scanning electron microscopy and confocal laser scanning microscopy also show that glutaraldehyde/HEMA causes serum protein coagulation (denaturation) that further blocks dentinal tubules.8
DENTAL ADHESIVES
Dentin-bonding agents also occlude dentinal tubules. Two randomized controlled trials found that adhesives significantly relieved DH for up to 1 month.9,10 A double-blind, randomized, parallel, prospective study compared the effectiveness of a glutaraldehyde/HEMA desensitizer, a dentin desensitizer/adhesive combination, and a self-etching adhesive in the chairside treatment of DH. All products demonstrated a reduction in DH compared with controls, but the glutaraldehyde/ HEMA desensitizer and the dentin desensitizer/adhesive combination resulted in the most significant improvements.11 Advancement in this area include the use of a light cure over a resin-based adhesive to inhibit the flow of fluid in the tubules for longer periods of time.
ARGININE-CALCIUM CARBONATE
A topical paste containing 8% arginine-calcium carbonate inhibits the hydrodynamic mechanism of DH by plugging patent tubules and has been shown to be clinically effective.12 In a study of 68 patients, a single professional application of arginine-calcium carbonate paste by prophylaxis cup provided statistically significant reductions in DH immediately, with results lasting for about a month.13 This product may help manage post-instrumentation discomfort in patients with a known history of DH.
LASERS
Research findings vary regarding the effectiveness of lasers in the clinical treatment of DH. The body of evidence supporting their use, however, is growing. Low-level output lasers, such as helium-neon and diode types, may function by interfering with nerve transmission. A systematic review and meta-analysis reported heterogeneity of results, with reported success rates ranging from 5% to 100%.14 The combination of low-level laser and glutaraldehyde/HEMA application can improve DH for up to 6 months.15 High-level output lasers, such as Nd:YAG and CO2 lasers, have also been evaluated in clinical studies. The Nd:YAG laser is thought to contract the dentinal tubules through the energy transferred by the laser, thereby reducing DH symptoms. According to a systematic review, the performance of lasers for relieving DH symptoms varies, but new studies report encouraging results.16 Additional well-designed clinical trials will help better demonstrate the value of lasers for managing DH.
OTHER THERAPIES
While potassium nitrate is a common ingredient in over-the-counter dentifrices, it is also available in at-home and in-office gels. Potassium nitrate increases extracellular potassium ion concentration, depolarizing the nerve and inhibiting it from repolarizing. The literature regarding its efficacy is mixed.17 Strontium salts work in the same manner as potassium nitrate, aiming to desensitize the nerve. Its secondary mechanism involves blocking patent dentin tubules.18
Calcium phosphate therapies—including amorphous calcium phosphate (ACP), casein phosphopeptide-ACP, bioactive glass, and tri-calcium phosphate—are also used to address DH. ACP, CPP-ACP, and tri-calcium phosphate provide additional calcium and phosphate ions to support remineralization of tooth structure, which may provide DH relief. Bioactive glass creates a hydroxycarbonate apatite layer to block tubules, thus decreasing sensitivity. These technologies are available as in-office gels, varnishes, prophylaxis pastes, professionally dispensed cream and gum, and prescription toothpastes. More in vivo research is needed to demonstrate their effectiveness in DH treatment.
PREVENTING DENTINAL HYPERSENSITIVITY
Oral health professionals should take care to avoid overinstrumenting exposed dentin with hand, ultrasonic, or polishing modalities in order to reduce the risk of DH. Once DH is reported, clinicians should attempt to determine its cause. Alternate etiologies for DH-like symptoms include caries, fractured/chipped teeth, and faulty restorations. Clinicians should consider the role of recent dental treatments, such as the timing of restorative procedures and scaling and root planing, that may cause sensitivity. Also, clinicians need to determine contributing etiological factors such as overzealous toothbrushing. Dietary counseling to manage erosion caused by acidic foods may be required. For example, an increased consumption of wine, acidic juices, or soft drinks can facilitate the dissolution of dentinal smear layers, thus, uncovering previously occluded dentinal tubules. When assessment fails to lead to the cause, patients can be asked to keep symptom diaries to help isolate specific etiologies. A short-term approach, prior to the clinical treatment of DH, may include the use of at-home prescription fluoride products and/or calcium phosphate therapies. If not successful, follow-up therapy may involve clinical assessments aimed at isolating symptomatic sites and treating them accordingly.
The current body of published clinical research does not offer overwhelming support for one treatment modality over another. In light of this, carefully developing a comprehensive approach that uses both at-home and in-office strategies based on the best available contemporary evidence is critical to effectively addressing DH in all patient populations.
References
- Gillam DG. Management of dentin hypersensitivity. Clin Oral Investig. 2015;2:87–94.
- Shiau HJ. Dentin hypersensitivity. J Evid Based Dent Pract. 2012;12(3 Suppl):220–228.
- Brannstrom M. The surface of sensitive dentine. an experimental study using replication. Odontol Revy. 1965;16:293–299.
- Ritter AV, de L Dias W, Miguez P, Caplan DJ, Swift EJ Jr. Treating cervical dentin hypersensitivity with fluoride varnish: A randomized clinical study. J Am Dent Assoc. 2006;137:1013–1020.
- Miller S, Truong T, Heu R, Stranick M, Bouchard D, Gaffar A. Recent advances in stannous fluoride technology: Antibacterial efficacy and mechanism of action towards hypersensitivity. Int Dent J. 1994;44(Suppl 1):83–98.
- Armenio RV, Fitarelli F, Armenio MF, Demarco FF, Reis A, Loguercio AD. The effect of fluoride gel use on bleaching sensitivity: A double-blind randomized controlled clinical trial. J Am Dent Assoc. 2008;139:592–597.
- Kakaboura A, Rahiotis C, Thomaidis S, Doukoudakis S. Clinical effectiveness of two agents on the treatment of tooth cervical hypersensitivity. Am J Dent. 2005;18:291–295.
- Ishihata H, Finger WJ, Kanehira M, Shimauchi H, Komatsu M. In vitro dentin permeability after application of gluma desensitizer as aqueous solution or aqueous fumed silica dispersion. J Appl Oral Sci. 2011;19:147–153.
- Ozen T, Orhan K, Avsever H, Tunca YM, Ulker AE, Akyol M. Dentin hypersensitivity: A randomized clinical comparison of three different agents in a short-term treatment period. Oper Dent. 2009;34:392–398.
- Yu X, Liang B, Jin X, Fu B, Hannig M. Comparative in vivo study on the desensitizing efficacy of dentin desensitizers and one-bottle self-etching adhesives. Oper Dent. 2010;35:279–286.
- Patil SA, Naik BD, Suma R. Evaluation of three different agents for in-office treatment of dentinal hypersensitivity: A controlled clinical study. Indian J Dent Res. 2015;26:38–42.
- Petrou I, Heu R, Stranick M, et al. A breakthrough therapy for dentin hypersensitivity: How dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teeth. J Clin Dent. 2009;20:23–31.
- Schiff T, Delgado E, Zhang YP, Cummins D, DeVizio W, Mateo LR. Clinical evaluation of the efficacy of an in-office desensitizing paste containing 8% arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity. Am J Dent. 2009;22:8A–15A.
- Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K. Treatment of dentine hypersensitivity by lasers: A review. J Clin Periodontol. 2000;27:715–721.
- Lopes AO, Eduardo Cde P, Aranha AC. Clinical evaluation of low-power laser and a desensitizing agent on dentin hypersensitivity. Lasers Med Sci. 2015;30:823–829.
- Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Lasers for the treatment of dentin hypersensitivity: A meta-analysis. J Dent Res. 2013;92:492–499.
- Addy M. Dentine hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:367–375.
- Pashley D. Dynamics of the pulpo-dentin complex. Crit Rev Oral Biol Med. 1996;7:104–133.
From Dimensions of Dental Hygiene. April 2016;14(04):18,20–21,23.

