LABORER/ISTOCK/GETTY IMAGES PLUS
LABORER/ISTOCK/GETTY IMAGES PLUS
Addressing Dentinal Hypersensitivity With Mouthrinse
Research shows that some formulations may offer patients relief from this common oral condition.
Dentinal hypersensitivity (DH) is one of the most common complaints among dental patients.1 It is defined as pain resulting from exposed dentin in response to chemical, thermal, tactile, or osmotic stimuli, which cannot be credited to any other type of dental pain.2 Previous research has shown the prevalence of DH ranges from 3% to 98%, depending on the diagnostic method used and the specific population studied.3,4 The most common age range of those affected is between 20 and 50, with DH incidence higher among women than men.5 Additionally, research has specified the facial aspect of the cervical margin of canines and first premolars to be the most often affected due to their prominent position in both the maxillary and mandibular arches.1,5
Etiology
Several theories are used to describe the etiology of DH. The direct innervation theory suggests that nerve endings enter the dentin through the pulp and extend to the dentinoenamel junction.6,7 Pain is directly signaled upon mechanical stimuli. Limited evidence suggests that nerves are in the superficial dentin; this theory does not have widespread support.
The odontoblast receptor theory suggests that odontoblasts act as receptors of pain and conduct signals to the pulpal nerves.6,7 However, this theory is not widely accepted due to the fact odontoblasts are not capable of producing nerve impulses.7
The most widely accepted theory concerning the development of DH is the hydrodynamic theory. Dentin is a porous tissue traversed by fluid-filled dentinal tubules.3 The hydrodynamic theory states that thermal, osmotic, or physical stimuli create movement of fluid within dentinal tubules, leading to the activation of nerve endings, known as baroreceptors, at the border of the dentin and pulp.1,7 The activation of baroreceptors causes a sharp and rapid pain leading to discomfort and sensitivity.1 Research has revealed that the tooth areas presenting sensitivity contain a larger number of exposed dentinal tubules, with a wider diameter, than the dentin in nonsensitive areas.3
DH is a complex condition associated with dentin exposure, open dentinal tubules, and nerve responsiveness to external stimuli.8 Clinical conditions thought to aid in the development of DH are enamel attrition, erosion, abrasion, and abfraction.8 Additionally, periodontal tissue loss (gingival recession) is another major predisposing factor because this can lead to exposure of cervical and root dentin.5,8
Diagnosis and Principles of Treatment
As like any other clinical condition, an accurate diagnosis is imperative to the effective management of DH. The diagnosis of DH can be tricky, as it has characteristics similar to other conditions such as caries, fractured or chipped enamel/dentin, pain due to reversible pulpitis, and dental bleaching sensitivity.5 Diagnosis of DH starts with a comprehensive dental history and clinical examination. The other causes of dental pain should be eliminated before a definitive diagnosis of DH is determined. Several techniques used to identify DH include the use of pure air, pure water, and sounds to mimic stimulation factors and determine the severity of symptoms.6 Additionally, palpation for diagnosing pulpitis or periodontal involvement, biting a wood stick, or transillumination for diagnosing a fractured or cracked tooth may also be used to rule out other causes of pain.5,6 DH treatment should begin with noninvasive options and progress to more invasive interventions if symptoms continue to progress. In-office management options may incorporate topical application of desensitizing agents, root exposure coverage, laser therapy, restorative treatment, and/or pulpal therapy.9
The diagnosis of dentinal hypersensitivity can be tricky, as it has characteristics similar to other conditions…
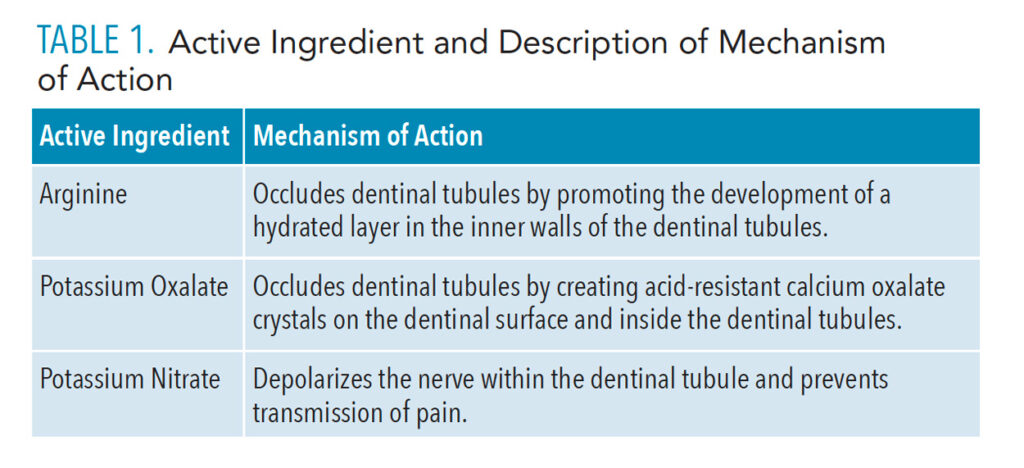
Based on the etiology linking dentin stimulation with pain, tooth sensitivity treatments can work in two ways to reduce symptoms. First, treatments can reduce dentin permeability and limit dentinal fluid shifts that activate the nerve by occluding the tubules.9 Second, treatments may decrease the excitability of the nerve, making the nerves unresponsive to stimulus, commonly referred to as depolarization.10 The following active agents found in desensitizing oral rinses promote the formation of surface structures that occlude the dentinal tubules or act to depolarize the nerve (Table 1).
![TABLE 1. Active Ingredient and Description of Mechanism of Action]() Clinical Efficacy of Desensitizing Oral Rinses
Clinical Efficacy of Desensitizing Oral Rinses
Commonly recommended treatment options for patients with DH include the use of desensitizing dentifrices. However, recent advances in the treatment of sensitivity may encourage the dental community to consider the use of desensitizing oral rinses. Oral rinses expose the entire oral cavity to the therapeutic agent, whereas the use of dentifrice limits the exposure of nontooth structures. The disadvantage of oral rinses is the need to deliver lower concentrations than would normally be delivered with a dentifrice.10 This is determined by safety considerations because if products are inadvertently swallowed, a greater volume of an oral rinse would be ingested compared to a dentifrice.10
Mello et al11 found an arginine oral rinse containing 0.8% arginine, polymethylvinyl ether/maleic acid (PVM/MA) copolymer, pyrophosphates, and 0.05% sodium fluoride demonstrated diffusion of a hydrated layer in the inner walls of the dentinal tubules. This hydrated layer formation indicated an affinity of the arginine for the dentin surface allowing for the dentinal tubules to be occluded reducing sensitivity.
Additionally, Hu et al12 evaluated the efficacy of an oral rinse containing 0.8% arginine, PVM/MA copolymer, pyrophosphates, and 0.05% sodium fluoride compared to a negative control group. Results indicated that after 8 weeks of product use, individuals in the arginine oral rinse group demonstrated statistically significant improvements in DH scores compared to the negative control group.
Further research is needed with longitudinal clinical trials comparing the use of desensitizing oral rinses with different active agents.
Another study done by Hall et al13 evaluated the efficacy of an experimental oral rinse containing 3% potassium nitrate (KNO3) in the treatment of DH when used as an adjunct to toothbrushing with fluoride toothpaste compared with the use of the same fluoride toothpaste alone. Potassium nitrate depolarizes the nerve within the dentinal tubules and prevents the transmission of pain.1 Results showed twice-daily use of a 3% KNO3 oral rinse, adjunctive to toothbrushing with fluoride toothpaste, provided significant improvements in DH compared with fluoride toothpaste alone.
In order to assess long-term relief from DH, Hall et al14 conducted a second study using the same parameters as their previous research on 3% KNO3 experimental oral rinse. Results found that adjunctive use of a 3% KNO3 oral rinse did not provide statistically significant improvements in DH for all clinical measures at each time point compared with use of fluoride toothpaste alone. However, the reduction in sensitivity documented was compatible with the findings of the previous study after 8 weeks of twice-daily use of 3% KNO3 oral rinse.
Moreover, results from previous research have shown that pastes or aqueous solutions containing potassium oxalate (KO) occlude dentinal tubules by creating acid-resistant calcium oxalate crystals on the dentinal surface and inside the dentinal tubules.15 This accumulation limits fluid movement and, as a result, reduces DH leading to discomfort or pain.16 A study conducted by Lynch et al16 demonstrated that KO oral rinse used as an adjunct to toothbrushing significantly controlled and reduced DH after twice-daily use for 4 weeks.
Furthermore, research conducted by Boneta et al17 evaluated the clinical efficacy of a dentifrice and oral rinse containing either arginine, potassium, or fluoride in reducing DH. Results showed the use of arginine provided the greatest reduction in tactile and air-blast DH compared to potassium and negative control regimens. Also, results showed arginine provided faster DH relief than potassium.
Conclusion
Although clinical studies have examined the effectiveness of desensitizing oral rinses, their use alone or as a supplement to dentifrice has not been thoroughly evaluated. Further research is needed with longitudinal clinical trials comparing the use of desensitizing oral rinses with different active agents. As more information becomes available, oral health professionals will have more treatment options to consider based on individual patient assessment and needs.
References
- Clark D, Levin L. Non-surgical management of tooth hypersensitivity. Int Dent J. 2016;66:249–256.
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–226.
- Molina A, García-Gargallo A, Montero E, et al. Clinical efficacy of desensitizing mouthwashes for the control of dentin hypersensitivity and root sensitivity: a systematic review and meta-analysis. Int J Dent Hyg. 2017;15:84–94.
- Osmari D, Fraga S, de Oliveira Ferreira AC, et al. In-office treatments for dentin hypersensitivity: a randomized split-mouth clinical trial. Oral Health Prev Dent. 2018;16:125–130.
- Miglani S, Aggarwal V, and Ahuja B. Dentin hypersensitivity: recent trends in management. J Conserv Dent. 2010;13:218–224.
- Davari A, Ataei E, Assarzadeh H. Dentin hypersensitivity: etiology, diagnosis and treatment; a literature review. J Dent Shiraz Iran. 2013;14:136–145.
- Haneet RK, Vandana LK. Prevalence of dentinal hypersensitivity and study of associated factors: a cross-sectional study based on the general dental population. Davangere, Karnataka, India. Int Dent J. 2016;66:49–57.
- Liu X, Tenenbaum H, Wilder RS, et al. Pathogenesis, diagnosis and management of dentin hypersensitivity: an evidence-based overview for dental practitioners. BMC Oral Health. 2020;20:220.
- Willershausen I, Schulte D, Azaripour A, et al. Penetration potential of a silver diamine fluoride solution on dentin surfaces: an ex vivo study. Clin Lab. 2015;61:1695–1701.
- Markowitz K. A new treatment alternative for sensitive teeth: a desensitizing oral rinse. J Dent. 2013;41(Suppl 1:)S1–S11.
- Mello SV, Arvanitidou E, Stranick MA, et al. Mode of action studies of a new desensitizing mouthwash containing 0.8% arginine, PVM/MA copolymer, pyrophosphates, and 0.05% sodium fluoride. J Dent. 2013;41(Suppl 1):S12–S19.
- Hu D, Stewart B, Mello S, et al. Efficacy of a mouthwash containing 0.8% arginine, PVM/MA copolymer, pyrophosphates, and 0.05% sodium fluoride compared to a negative control mouthwash on dentin hypersensitivity reduction. A randomized clinical trial. J Dent. 2013;41(Suppl 1):S26–S33.
- Hall C, Sufi F, Milleman JL, et al. Efficacy of a 3% potassium nitrate mouthrinse for the relief of dentinal hypersensitivity: An 8-week randomized controlled study. J Am Dent Assoc. 2019;150:204–212.
- Hall C, Sufi F, Constantin P. Efficacy of an experimental 3% potassium nitrate mouthwash in providing long-term relief from dentin hypersensitivity: an 8-week randomized controlled study (Study 2). Am J Dent. 2017;30:335—342.
- Sauro S, Gandolfi MG, Prati C, Mongiorgi R. Oxalate-containing phytocomplexes as dentine desensitizers: an in vitro study. Arch Oral Biol. 2006;51:655–664.
- Lynch MC, Perfekt R, McGuire JA, et al. Potassium oxalate mouthrinse reduces dentinal hypersensitivity: A randomized controlled clinical study. J Am Dent Assoc. 2018;149:608–618.
- Boneta ARE, Ramirez K, Naboa J, et al. Efficacy in reducing dentine hypersensitivity of a regimen using a toothpaste containing 8% arginine and calcium carbonate, a mouthwash containing 0.8% arginine, pyrophosphate and PVM/MA copolymer and a toothbrush compared to potassium and negative control regimens: an eight-week randomized clinical trial. J Dent. 2013;41:S42–S49.
From Dimensions of Dental Hygiene. January 2022;20(1):21-23.