
A New Model of Periodontal Susceptibility
As our knowledge of the matricellular events that shift the path from periodontal health to disease grows, treatments that can be implemented before symptoms ever appear may become possible.
This course was published in the October 2012 issue and expires October 2015. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the importance of understanding the molecular and cellular events that lead to periodontal diseases.
- Explain the roles played by the different molecules present in the periodontium and how they affect periodontal health.
- Describe the role of periostin as a possible indicator of genetic susceptibility.
INTRODUCTION
New data on the prevalence of periodontitis in the United States have recently been published in the Journal of Dental Research by Eke et al. The information comes from the 2009-2010 National Health and Examination Survey and reports that 47.2% of adults have mild, moderate, or severe periodontitis. This shocking increase in the prevalence of periodontitis compared to previous estimates is likely due to the full mouth examinations used in the 2009-2010 survey vs the partial mouth estimates that were conducted in previous surveys. This article, “A New Model of Periodontal Susceptibility” provides a timely update to our understanding of the molecular and cellular events involved in periodontal diseases, as they potentially impact almost half of our adult patients. In addition, new research in the area of genetic susceptibility is reviewed. The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support the last article of this four part series in collaboration with the American Academy of Periodontology.
—Barbara Shearer, BDS, MDS, PhD, Director of Scientific Affairs, Colgate Oral Pharmaceuticals
PERIODONTAL DISEASES CURRENTLY AFFECT MORE THAN 40% OF PEOPLE IN THE UNITED STATES, WITH 38% PRESENTING WITH MODERATE TO SEVERE DISEASE STATUS.1 HOWEVER, today’s treatment protocols are limited by the fact that treatment cannot begin until signs and symptoms emerge, which may be after normal tissue function is altered or lost. In order to achieve better outcomes, the molecular and cellular events that lead to periodontal diseases need to be better understood. The identification of genetic susceptibility variants and their role in disease onset and progression are currently under investigation. If more was known about the preclinical molecular events happening during the genesis of periodontal diseases, identifying patients at increased risk could be possible, which may ultimately lead to successful intervention before signs and symptoms appear.
MICROBIAL CHALLENGES
Complex periodontal pathogenic biofilm in a susceptible host is the primary etiology of periodontal diseases.2 The current standard of care identifies, diagnoses, and treats periodontal diseases based on distinct clinical features that support a common treatment protocol. However, the clinical reality reflects the heterogeneity and complexity of the factors that influence the host’s susceptibility and, subsequently, the pathogenesis of periodontal diseases. Deciphering the biologic phenotype that determines tissue function and structure within an individual is important.3 However, acknowledging that there are various compensatory biological mechanisms that could divert the tissue phenotype and, ultimately, determine proper tissue homeostasis and health from individual to individual is imperative. Numerous investigations are geared toward bridging this knowledge gap in the periodontal pathogenesis field to support the development of practical and predictable applications for the diagnosis, treatment, and prevention of periodontal diseases.
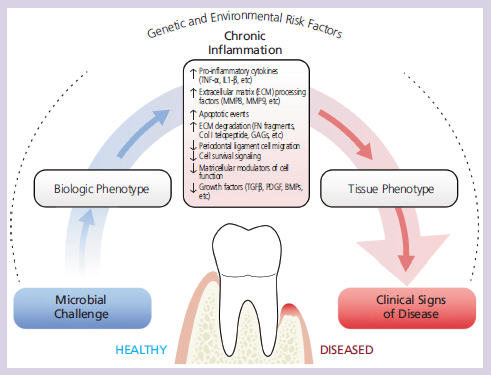
The proper regulation of these interactions, while under constant periodontal microbial challenge, determines the biologic phenotype that ultimately favors or prevents tissue homeostasis.14,15 Throughout life, the periodontal homeostasis is challenged by genetic and environmental factors. In a susceptible host, the periodontal pathogenic biofilm triggers a chronic inflammatory response that causes significant matricellular molecular changes and favors periodontal disease progression. An increase of proinflammatory cytokines that supports the degradation and processing of important structural molecules characterize the transition from tissue health to disease.16,17 These associated protein fragments exert a specific biological function that weakens the extracellular matrix and induces apoptotic events. This altered biologic phenotype disrupts normal matricellular function that diminishes cell proliferation and migration—important processes for adequate tissue turnover.18 Altered levels of important growth factors have been implicated in advanced periodontal diseases.16–19 Collectively, these molecular changes compromise the integrity of the periodontium, which translates into an altered tissue phenotype that, ultimately, leads to the appearance of the clinical signs and symptoms of established periodontal disease.
DYNAMICS OF PERIODONTAL DISEASE PATHOGENESIS
In a healthy individual, specific bacteria species associate and form complexes that coexist with the host. In periodontal diseases, the subgingival microflora shifts, and Gram-negative anaerobes become predominant—challenging the tissue integrity.2,4 Epithelial and connective tissue structures, such as the junctional epithelium and the periodontal ligament (PDL), represent the first line of defense against the constant physical and microbial challenges. However, different environmental and genetic factors alter the host’s ability to sustain such challenges, which supports the theory that the bacterial plaque in a susceptible host is the primary etiologic factor in periodontal diseases.
Like gingivitis, periodontal diseases are characterized by a lymphocytic and plasma cell infiltrate that is driven by a rich cytokine (cell signaling protein molecules used in intercellular communication) environment.5 Periodontitis is clinically different from gingivitis because it results in connective tissue attachment loss in the presence of chronic inflammation. Despite the histopathological similarities between gingivitis and periodontitis, the mechanism that determines the progression of gingivitis to periodontitis and precipitates the loss of connective tissue integrity and, subsequently, loss of attachment is not completely understood. The periodontium undergoes molecular biology changes in response to bacteria and chronic periodontal inflammation. Understanding this sequence of events illustrates important clinical implications that may help determine disease activity while providing insight into the molecular networks behind patients’ susceptibility to periodontal breakdown.
Major components of the PDL extracellular matrix (intercellular substance from which a structure develops) are processed during the initiation and progression of periodontal diseases, resulting in the solubilization of detectable fragments. Inflammation-associated levels of extracellular matrix (ECM) fragments, growth factors, and proteases are measurable in periodontal tissues, gingival crevicular fluid (GCF), and saliva.5,6 Exploration of these molecules have provided an understanding of disease-associated ECM changes in the active periodontal lesion. Pioneer work in this area reported a significant increase in the level of glysosaminoglycans in the GCF of periodontal disease-affected sites.7 Increased levels of pyridinoline cross-linked carboxyterminal telopeptide of type I collagen fragments also correlate with active periodontal bony destruction.8
Some of the breakdown products may exert specific functions. The pro-apoptotic 40kD fibronectin (FN) fragment is an example of an inflammation-associated product with specific bioactivity.9,10 The 40kD FN fragment is elevated in severe cases of periodontitis and appears to induce the disruption of cell-matrix interactions by the cell-detachment- induced apoptosis (process of programmed cell death)—commonly refered to as anoikis.11
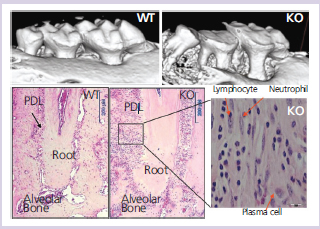
Recently, with advances in technology and the availability of preclinical models, a more mechanistic characterization of the disease process appears feasible (Figure 1).12–19 Deletion of specific target genes allows an in vivo validation of the importance of certain proteins in the context of periodontal tissue homeostasis and repair.
The genetically modified animal models allow a focused evaluation of specific structures in vivo; for example, the evaluation of the sulcular epithelium, an important structural tissue barrier, that when breached by oral bacteria, triggers a robust immunological defense reaction. This inflammatory reaction may be contained at this level or, as in the case of susceptible individuals, it may progress to compromise the PDL integrity and the alveolar bone. The molecule ?v?6 integrin is expressed in the healthy junctional epithelium. Mice lacking the transmembrane protein ?v?6 integrin lose rapid attachment and a significant apical migration of the sulcular epithelium favors the formation of periodontal pockets.20 These changes are associated with altered signaling of TGF?-1, a molecule involved in multiple regulatory functions, including tissue repair and immune function. TGF?-1 is secreted from the cells in the healthy periodontium as a latent precursor complex. The secreted latent TGF?-1 is bound to the ECM via a latent TGF?-1 binding protein (LTBP1).21 Binding of ?v?6 integrin to the latent-associated propeptide has been implicated in the activation and release of the latent TGF?-1, which in turns favors ECM formation and maturation by the regulation of MMP-8 and MMP-13.16,22 The examination of tissues from patients with advanced periodontal disease shows consistent changes characterized by decreased TGF?-1 and ?v?6 integrin, while also demonstrating increased levels of tissue collagenases, gelatinases, and proinflammatory cytokines.17,23
Another example of these genetically-modified animal models is the periodontal phenotype resulting from the genetic deletion of the TGF?-regulated PDL marker known as periostin.18 In the absence of periostin, the integrity of the periodontal structures is severely compromised (Figure 2).
Collectively, these findings may significantly impact current understanding of periodontal cell-matrix dynamics and homeostasis, which may ultimately help uncover new pathways of ascertaining disease susceptibility. These pathways could then be targeted in the prevention and treatment of periodontal diseases. One of the long-term goals of this approach includes developing practical therapeutic protocols that help repair and regenerate diseased periodontium.
IDENTIFYING A POTENTIAL NOVEL TARGET
The selection of specific targets and their role within this complex periodontal environment is just as important as identifying the molecular milieu relevant to health and disease. An ideal target/biomarker should be highly specific, highly sensitive, biologically stable for feasible detection, predictive (eg, proportionate to extent of injury), and noninvasive/accessible (eg, measurable in retrievable samples). The identification of genetic susceptibility variants and their role in disease onset and progression are fundamental to identifying new determinants of periodontal stability.
Currently, periodontal conditions are diagnosed based on the clinical presentation of the disease. The current classification identifies “different” forms of the disease that manifest with a common clinical presentation and clusters them within groups (eg, chronic, aggressive, necrotizing). The lack of a biology-based classification system prevents the establishment of more homogeneous diagnosis categories and more predictable treatment outcomes.15 A molecular-based model for periodontal disease patho genesis could help explain the host’s susceptibility to periodontal breakdown by capturing the dynamic nature of the matricellular biochemical events involved in the transition between periodontal health and disease. Periostin is one of the most important modulators of cell-matrix interactions and a known PDL marker. Recent preclinical models have reported on the importance of periostin in the maintenance of the periodontium’s function and integrity.18,24 Among the known proteins expressed in the PDL, periostin shows the greatest specificity. Periostin interacts with extracellular structural molecules and cell membrane proteins. These matricellular interactions influence cell behavior, as well as collagen fibrillogenesis.25 Periostin’s high specificity is particularly valuable, and supports a promising diagnostic potential for this molecule.
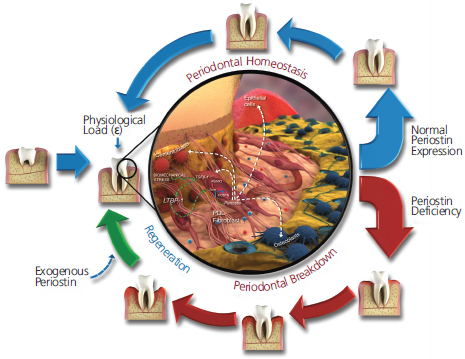
Periostin is necessary for PDL function and proper periodontal integrity. Periostin is also decreased by the effect of pro-inflammatory cytokines, microbial virulence factors, or a combination of both.26,27 The presence of mechanical challenge makes those deleterious effects even worse. Decreased periostin protein levels are noticed in vitro in the presence of bacteria and inflammatory mediators.
A recent publication using a preclinical periodontitis ligature model further supports the potential association of periostin in the pathogenesis of periodontal diseases.27 Chronic periodontal inflammation in this model resulted in a clear correlation between decreased periostin levels and detrimental changes to the periodontium over time. When periostin is reduced or completely depleted, the stability of the PDL is compromised, making it more susceptible to the inflammatory environment as its structural and biomechanical properties are reduced.18 Such mechanism supports the idea of a complex regulatory pathway of periodontal tissue homeostasis where periostin is a key factor in maintaining tissue integrity under biomechanical, bacterial, and inflammatory challenges (Figure 3).
Understanding these molecular dynamic events in the context of chronic periodontal inflammation should go beyond the simple characterization of this phenomenon and be translated into promising diagnostic and therapeutic alternatives to provide more predictable and individualized patient care.
CONCLUSION
Molecules, such as periostin, serve as potential novel biomarkers that may aid in the understanding of periodontal biology, cell-matrix dynamics, and homeostasis. Collectively, the available evidence and evolving understanding of the molecular dynamics that determine a patient’s susceptibility to periodontal breakdown, triggered by periodontal pathogens, would assist clinicians in the complex task of identifying important etiologic contributors that could be targeted in the treatment of inflammatory periodontal diseases.
ACKNOWLEDGEMENTS
The author would like to thank Chris Jung and Kenneth Rieger for their help with the figures.
This research was supported by NIH/NIDCR grant K23DE019872. LAGUNA DESIGN/PHOTO RESEARCHERS INC This course was developed in part with an unrestricted educational grant from Colgate-Palmolive Company and in collaboration with the American Academy of Periodontology.
REFERENCES
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalenceof periodontitis in adults in the United States: 2009 and 2010. JDent Res. 2012;91:914–920.
- Socransky SS, Haffajee AD, Smith C, et al. Use of checkerboardDNA-DNA hybridization to study complex microbial ecosystems.Oral Microbiol Immunol. 2004;19:352–362.
- Offenbacher S, Barros SP, Beck JD. Rethinking periodontalinflammation. J Periodontol. 2008;79:1577–1584.
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL, Jr.Microbial complexes in subgingival plaque. J Clin Periodontol.1998;25:134–144.
- Listgarten MA. Pathogenesis of periodontitis. J Clin Periodontol.1986;13:418-430.
- Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkersfor oral and periodontal diseases. Dent Clin North Am.2005;49:551 –571.
- Giannobile WV, Riviere GR, Gorski JP, Tira DE, Cobb CM. Glycosaminoglycansand periodontal disease: analysis of GCF bysafranin O. J Periodontol. 1993;64:186–190.
- Giannobile WV, Lynch SE, Denmark RG, Paquette DW, FiorelliniJP, Williams RC. Crevicular fluid osteocalcin and pyridinoline crosslinkedcarboxyterminal telopeptide of type I collagen (ICTP) asmarkers of rapid bone turnover in periodontitis. A pilot study inbeagle dogs. J Clin Periodontol. 1995;22:903–910.
- Jee SW, Wang S, Kapila YL. Specific pro-apoptotic fibronectinfragments modulate proteinase expression in periodontal ligamentcells. J Periodont. 2004;75:523–530.
- Huynh QN, Wang S, Tafolla E, et al. Specific fibronectin fragmentsas markers of periodontal disease status. J Periodontol.2002;73:1101–1110.
- Dai R, Iwama A, Wang S, Kapila YL. Disease-associatedfibronectin matrix fragments trigger anoikis of human primary ligamentcells: p53 and c-myc are suppressed. Apoptosis.2005;10:503–512.
- Gotz W, Heinen M, Lossdorfer S, Jager A. Immunohistochemicallocalization of components of the insulin-like growth factorsystem in human permanent teeth. Arch Oral Biol.2006;51:387–395.
- Reichenberger E, Baur S, Sukotjo C, Olsen BR, Karimbux NY,Nishimura I. Collagen XII mutation disrupts matrix structure ofperiodontal ligament and skin. J Dent Res. 2000;79:1962–1968.
- Yang YQ, Li XT, Rabie AB, Fu MK, Zhang D. Human periodontalligament cells express osteoblastic phenotypes under intermittentforce loading in vitro. Front Biosci. 2006;11:776–781.
- Long P, Liu F, Piesco NP, Kapur R, Agarwal S. Signaling bymechanical strain involves transcriptional regulation of proinflammatorygenes in human periodontal ligament cells in vitro. Bone.2002;30:547–552.
- Yan C, Boyd DD. Regulation of matrix metalloproteinase geneexpression. Cell Physiol. 2007;211:19–26.
- Agarwal S, Chandra CS, Piesco NP, Langkamp HH, Bowen L,Baran C. Regulation of periodontal ligament cell functions by interleukin-1beta. Infect Immun. 1998;66:932–937.
- Rios HF, Ma D, Xie Y, et al. Periostin is essential for the integrityand function of the periodontal ligament during occlusal loadingin mice. J Periodontol. 2008;79:1480–1490.
- Atilla G, Emingil G, Kose T, Berdeli A. TGF-beta1 gene polymorphismsin periodontal diseases. Clin Biochem. 2006;39:929–934.
- Ghannad F, Nica D, Fulle MI, et al. Absence of alphavbeta6integrin is linked to initiation and progression of periodontal disease.Am J Path. 2008;172:1271–1286.
- Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforminggrowth factor-beta 1 associates to fibroblast extracellularmatrix via latent TGF-beta binding protein. J Cell Biol.1994;124:171–181.
- Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGFbetabinding protein-1. J Cell Biol. 2004;165:723–734.
- Silva N, Dutzan N, Hernandez M, et al. Characterization of progressiveperiodontal lesions in chronic periodontitis patients: levelsof chemokines, cytokines, matrix metalloproteinase-13,periodontal pathogens and inflammatory cells. J Clin Periodontol.2008;35:206–214.
- Hamilton DW. Functional role of periostin in development andwound repair: implications for connective tissue disease. J CellCommun Signal. 2008;2:9–17.
- Norris RA, Damon B, Mironov V, et al. Periostin regulates collagenfibrillogenesis and the biomechanical properties of connectivetissues. J Cell Biochem. 2007;101:695–711.
- Padial-Molina M, Volk SL, Rodriguez JC, Marchesan JT, Galindo-Moreno P, Rios HF. TNF-Alpha and P. gingivalis LipopolysaccharidesDecrease Periostin in Human PDL Fibroblasts. J Periodontol. 2012Jul 6. [Epub ahead of print].
- Padial-Molina M, Volk SL, Taut AD, Giannobile WV, Rios H.Periostin is downregulated during periodontal inflammation. J DentRes. 2012 Aug 29. [Epub ahead of print].
From Dimensions of Dental Hygiene. October 2012; 10(10): 19-22.



