Advancements in Tooth Whitening
The demand for whiter teeth has spurred the development of many innovations in professional oral health care technologies, as well as in over-the-counter (OTC) products. Discoloration can present in various ways, and, depending on the nature of the discoloration, the treatment approach and outcome can vary significantly.
Evolution of Esthetics
The demand for whiter teeth has spurred the development of many innovations in professional oral health care technologies, as well as in over-the-counter (OTC) products. Discoloration can present in various ways, and, depending on the nature of the discoloration, the treatment approach and outcome can vary significantly. When discussing tooth whitening, it is important to acknowledge the work of many pioneers who enabled the profession to offer tooth whitening as a conservative, alternative treatment to whiten and lighten discolored teeth that otherwise would have to be treated with veneers or crowns—with concomitant removal of sound tooth structure. Historically, professional whitening procedures were performed in the dental office, typically using concentrated hydrogen peroxide and by isolating surrounding soft tissue to avoid contact with the whitening agent. Although this proved effective, drawbacks included lengthy chairtime, cost, and the risk of dentinal hypersensitivity. These downsides were addressed by Haywood and Heymann in 1989, with the introduction of nightguard vital bleaching using 10% carbamide peroxide in a custom tray worn at night. This technique offered the possibility of whiter vital teeth at a reduced cost, and with fewer side effects. It also marked an evolutionary step in tooth whitening, as the patient took on the responsibility of performing the procedure at home.
Photo Credit: MartinPrescott / E+
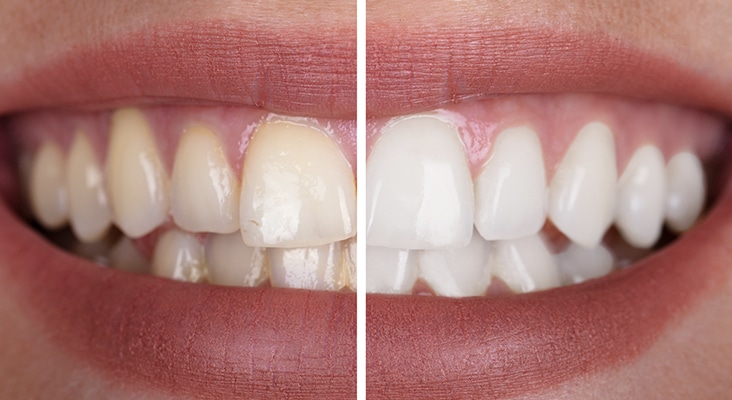
Tooth Discoloration
The etiology of tooth discoloration is broadly classified as extrinsic or intrinsic, depending on the origin of the stain. It has also been reported that long-standing extrinsic stains can become internalized, making removal more challenging. Extrinsic stains of various color—such as those caused by coffee, tea, red wine, tobacco, and colored food—are caused by superficial accumulation of residue on the enamel surface. These can be accentuated by pitting or irregularities of the enamel, salivary composition, salivary flow rates, and poor oral hygiene. Stains remain on the tooth surface due to attractive forces, including long-range interactions (such as electrostatic and van der Waals forces), and short-range interactions (such as hydration forces, hydrophobic interactions, dipole-dipole forces, and hydrogen bonds).
Photo Credit: AndreyPopov / iStock / Getty Images Plus

Stain Removal
Traditionally, stains of extrinsic origin were removed by toothbrushing or dental prophylaxis, which relies on abrasive action. Stains of intrinsic origin, by comparison, could be removed only by oral health professionals using oxidizing agents, such as hydrogen or carbamide peroxide. There has been a paradigm shift, however, with two major events taking place. The first is the incorporation of oxidizing agents into OTC whitening products, as well as dentifrices. The second development is emerging evidence that extrinsic stains can become internalized, which makes a strict distinction between extrinsic and intrinsic discoloration more difficult to establish. One might argue, therefore, whether every type of discoloration eventually can be treated with self-applied methods on a daily basis.
Photo Credit: Georgijevic / E+

Safety of Abrasives
Extensive research has been conducted in optimizing the stain removal potential of dentifrice abrasives, while minimizing any deleterious wear effects to the tooth surface.13 The relative safety of abrasives is established by standard methods referred to as relative dentin abrasivity. A study comparing dentifrices showed that, with few exceptions, dentifrices marketed as whitening products were generally more abrasive to dentin, especially those containing silicas. The stain-removal potential of dentifrices in clinical trials are usually evaluated with common indexes, including the Lobene Stain Index or Macpherson-modified Lobene Stain Index, which are related to the area and intensity of the stain.
Photo Credit: scyther5 / iStock / Getty Images Plus
Over-the-Counter Options
OTC whitening products provide consumers with a variety of materials and delivery methods, typically at less cost than in-office or professionally dispensed, at-home whitening therapies. Among the systems available, strip technology generally contains hydrogen peroxide as the active ingredient. In concentrations not exceeding 10%, it has been reported to be safe and effective. Earlier concerns of not attaching to the tooth properly, and inability to cover a wide arch, are being addressed with new seal technology. In vitro studies have shown that when applied on the outer enamel surface, the hydrogen peroxide in strips was able to reach the pulp cavity, demonstrating that the active agent diffuses into the enamel and dentin to interact with stain molecules deep within the tooth structure.
Photo Credit: AndreyPopov / iStock / Getty Images Plus

Dentist-Dispensed Whitening Strategies
Dentist-dispensed, patient-applied home whitening is a well-established whitening procedure. It allows proper diagnosis, treatment planning, and professional supervision. It is relatively easy to perform, and may be less expensive than in-office whitening. Initially, it was used with 10% carbamide peroxide during nighttime bleaching sessions. A study of the kinetics of carbamide peroxide showed that it remains active for up to 10 hours, with about half of the active agent used up in the first 2 hours. Concentrations indicated for at-home whitening range between 10% to 35%. A 10% carbamide peroxide solution breaks down into 3.35% hydrogen peroxide and 6.65% urea. Urea further breaks down into ammonia and water, and may provide beneficial side effects—including slowing the caries process by increasing the pH value of the solution.
Photo Credit: klebercordeiro / iStock / Getty Images Plus

In-Office Whitening
In-office whitening is the initial form of tooth whitening, and is performed with hydrogen peroxide-based materials at concentrations of up to 40%. It is an alternative for patients who cannot tolerate trays, or who desire an instant whitening outcome and prefer to have the procedure performed in the office. A study that evaluated the time required to achieve a six-tab difference on a Vita Classical shade guide found that in-office whitening produced the fastest results, followed by professionally supervised at-home whitening. The researchers noted that OTC whitening required the most time. That said, the use of high-concentration hydrogen peroxide materials associated with in-office whitening also resulted in generally higher incidence of dentinal hypersensitivity. Recently, manufacturers have attempted to address this issue by reducing concentration levels and changing the delivery method from gels to varnish systems.
Photo Credit: AndreyPopov / iStock / Getty Images Plus

Preventing Hypersensitivity
In order to address the possible concerns of demineralization associated with whitening, several remineralization strategies using fluoride and calcium phosphate technologies have been suggested. Remineralization is the process whereby calcium and phosphate ions are supplied from a source externally to the tooth to promote ion deposition into the crystal voids in demineralized enamel to produce mineral gain. Agents to promote remineralization, as well as reduce tooth sensitivity after whitening include fluoride, potassium nitrate, amorphous calcium phosphate (ACP), casein phosphopeptide-ACP, calcium sodium phosphosilicate, arginine calcium carbonate, and tri-calcium phosphate. Whether these active components truly assist in remineralization after whitening has yet to be determined. However, the application of topical fluoride post-whitening has been demonstrated to be effective in restoring the mineral content and microhardness values back to the baseline level.

