Maintaining Equilibrium
Dimensions speaks with James S. Wefel, PhD, about how to maintain balance in the mouth’s remineralization/demineralization process.
Q. Can you briefly explain the process of remineralization?
A. . Essentially, the remineralization process is the opposite of demineralization. The two processes work in a dynamic equilibrium. In demineralization, mineral is lost from the tooth. In remineralization, we are trying to add mineral back, hopefully into the same spot from which it left. This doesn’t always happen but it is the most ideal type of remineralization because it makes the mineral less soluble and provides a higher grade of mineral than what was initially present.
Any time a solution is undersaturated, the solid will begin to erode. Enamel starts to dissolve when the oral fluid is undersaturated. If the oral fluid is saturated, then the enamel is at equilibrium with the solution. Supersaturation provides the ability for remineralization.
Fermentable carbohydrates from the diet feed the acidogenic microflora present in the mouth and organic acid is then produced. These acids change the pH of the oral fluid so that the oral cavity is no longer saturated, opening the door to demineralization.
 Enamel is called apatite but it is really a calcium-deficient carbonate-containing apatite. If the tooth can be remineralized and the carbonate, magnesium, and other impurities are disposed of, a better formed solid is created. The incorporation of fluoride creates a fluoridated hydroxyapatite, which is even less soluble.
Enamel is called apatite but it is really a calcium-deficient carbonate-containing apatite. If the tooth can be remineralized and the carbonate, magnesium, and other impurities are disposed of, a better formed solid is created. The incorporation of fluoride creates a fluoridated hydroxyapatite, which is even less soluble.
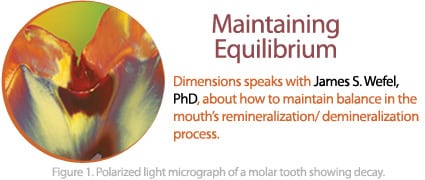
In healthy mouths, there is a constant balancing act between remineralization and demineralization. It’s only when the scales are tipped toward demineralization that white spot lesions occur and then, if the balance is consistently moved toward demineralization, frank cavitation results (Figure 1).
Q. What tips the scale toward demineralization?
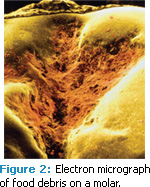
A. Demineralization occurs when bacteria metabolize fermentable carbohydrates into an acid, which then dissolves the tooth. Demineralization of tooth structure is caused by plaque that is not removed from the teeth and frequent daytime snacking with fermentable carbohydrates. The consistent exposure to fermentable carbohydrates that are not rinsed or brushed away allows acid to form and dissolve the enamel until saliva has the opportunity to buffer the acid and clear it from the mouth (Figure 2). Demineralization begins when the oral pH drops below the range of 5.6 to 5.4, which is the point at which undersaturation is reached.
The natural defenses provided by saliva can be helped by brushing, flossing, and limiting the number of times fermentable carbohydrates are ingested. If a patient eats three square meals a day in addition to 10 snacks, he or she is not giving the mouth much time to recover and remineralize from those demineralizing episodes.
Q. The US Department of Health and Human Services has revised the guidelines for water fluoridation. Can you explain why the recommended level of fluoride was changed to the lowest end of the previous optimal range?
A. First, we need to understand why there was a range for water fluoridation in the first place. Originally, water fluoridation guidelines incorporated the difference between average mean daily air temperatures in the northern United States and those in the southern states because it was thought that people in colder climates drank less water than those living in warmer areas. A range of fluoride exposure was created to accommodate possible regional differences in water consumption.7 This is how the initial range began with 0.7 ppm to 1.1 ppm of fluoride. This purported difference in drinking habits is now considered negligible so the levels have been set at the low end of the range.
 Data show that we most likely don’t need any more fluoride than 0.7 ppm.8 With more areas becoming fluoridated, we have a halo effect, which is where there are more possibilities of fluoride exposure in nonfluoridated areas from food and drinks being produced in areas that have fluoridated water. This “new level” hardly represents much of a change, it’s rather an adjustment to the lower end of the scale.
Data show that we most likely don’t need any more fluoride than 0.7 ppm.8 With more areas becoming fluoridated, we have a halo effect, which is where there are more possibilities of fluoride exposure in nonfluoridated areas from food and drinks being produced in areas that have fluoridated water. This “new level” hardly represents much of a change, it’s rather an adjustment to the lower end of the scale.
Exposure to too much fluoride causes fluorosis and there has been a rise in mild to moderate cases of fluorosis, most likely due to the halo effect. Most cases of fluorosis result only in cosmetic effects, however, such as opaque white areas on the enamel.9 Water fluoridation has been one of the most important achievements in public health because of its role in decreasing the prevalence of caries.10
Q. Besides fluoride, new remineralization technologies are currently available. Can you explain how these work?
A. The calcium phosphate-containing products are what I call salivary enhancers. These ions are incorporated into a variety of products, which claim to boost remineralization (see Table 1). All of them have the same objective: increase calcium phosphate in the oral environment. In order to meet this objective, the product must remain in the mouth, whether on plaque, teeth, or soft tissue, and not be washed away but rather freed up to have calcium phosphate available at the right time to either prevent demineralization or enhance remineralization.
In my opinion, the patients who will benefit most from these products are those with some lack of salivary flow or absence of appropriate salivary function. Saliva normally provides enough calcium phosphate to help buffer the teeth and to be available for remineralization. It’s only when the pH changes in the mouth that demineralization conditions occur. Healthy mouths maintain an equilibrium between demineralization and remineralization and have sufficient calcium and phosphate.
These salivary enhancers are good for people who have problems with salivary flow, such as those with xerostomia, which can be caused by disease, medications, and illicit drug use. Xerostomic patients do not have enough calcium phosphate and the enhancers can help tip the scales toward remineralization. Whether they are products that should be used by general populations is not well understood. More clinical studies are necessary to illustrate whether they are helpful to people with healthy mouths.
The views expressed in this interview are those of James S. Wefel, PhD.
REFERENCES
- Chow L, Wefel JS. The science of remineralization. Dimensions of Dental Hygiene. 2009;7(2):42-46.
- Hurlbutt M. Caries management with calcium phosphate. Dimensions of Dental Hygiene. 2010;8(10):40-46.
- Geiger S, Matalon S, Blasbalg J, Tung M, Eichmiller FC. The clinical effect of amorphous calcium phosphate (ACP) on root surface hypersensitivity. Oper Dent. 2003;28:496-500.
- Burwell AK, Litkowski LJ, Greenspan DC. Calcium sodium phosphosilicate (NovaMin): remineralization potential. Adv Dent Res. 2009;21:35-39
- Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587-1595.
- Karlinsey RL, Mackey AC. Solid-state preparation and dental application of an organically modified calcium phosphate. J Mater Sci. 2009;44:346-349.
- Bailey W, Barker L, Duchon K, Maas W. Populations receiving optimally fluoridated public drinking water—United States, 1992–2006. MMWR Morb Mortal Wkly Rep. 2008;57:737-741.
- Wong HM, McGrath C, Lo EC, King NM. Association between developmental defects of enamel and different concentrations of fluoride in the public water supply. Caries Res. 2006;40:481-486.
- Tinanoff N. Use of fluoride. In: Berg JH, Slayton R, eds. Early Childhood Oral Health. Hoboken, NJ: Wiley- Blackwell; 2009.
- Petersen PE, Lennon MA. Effective use of fluorides for the prevention of dental caries in the 21st century: the WHO approach. Community Dent Oral Epidemiol. 2004;32:319-321.
From Dimensions of Dental Hygiene. May 2011; 9(5): 42, 44, 46.

