Contaminated Surfaces
A look at multidrug-resistant organisms and computer keyboard/mouse devices in the dental office.
This course was published in the June 2009 issue and expires June 2012. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Understand that multidrug-resistant organisms (MDROs) are present in the dental environment.
- Describe the potential for cross-contamination within the dental office.
- Suggest methods to avoid acquiring or transmitting pathogens.
Multidrug-resistant organisms (MDROs), which have been a concern in hospitals and chronic care facilities for years,1-4 are now found in the general community—outside of medical health care environments.5-7 Preventing crosscontamination and identifying the source of these pathogens are key in reducing exposure to and transmission of these organisms. MDROs may reside in sites within the dental office that are not normally considered for decontamination. Recognizing potential sources of contamination, following basic hygiene precautions, and using barrier procedures can reduce the risk of MDRO transmission to dental patients and dental health care providers.
The recent outbreak of the H1N1 flu (swine flu) highlights the necessity of maintaining effective infection control protocols. For recommendations specific to the H1N1 flu, visit the Organization for Safety and Asepsis Procedures’ (OSAP) website at www.osap.org.
THE CONCERN
MDROs, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and certain gram-negative bacilli (GNB), can be found in the dental practice. Although MDROs most frequently appear as acquired infections in hospitals and acute care facilities,1 they have become increasingly reported in the community5,9 where they can trigger serious health problems, and may even cause death.5,9,17,18 A nosocomial infection, which is secondary to a patient’s original condition, develops when a patient receives treatment in a clinic or hospital setting.1-5 Nosocomial infections play a significant role in the growth of health care costs and morbidity in health care facilities. Many organisms found in nosocomial infections are MDROs.
MRSA is a resistant and virulent organism.2,3 This gram-positive bacterium is common among hospitalized patients who are immune suppressed, as well as the elderly and the very young. Patients who undergo surgery and those with indwelling devices, such as intravenous lines, drains, catheters, or nasal tracheal tubes, are especially at risk. MRSA frequently lives on the skin and in the nasal passages of patients and health care workers without causing health problems. These bacteria can be spread from person to person through casual contact or through contaminated objects or surfaces.
In 2005 an estimated 94,360 cases of MRSA infection occurred in the United States. More than 18,000 deaths are caused annually by MRSA infections.9 MRSA can be found in healthy people living in the general community. These bacteria can cause infections in nonhospitalized, healthy individuals who have cuts or wounds and have close contact with others.6-9 Recently, several deaths have been reported from community- acquired MRSA infections.9,17,18
Dental patients may carry a MDRO, which could conceivably be transmitted to dental office staff or other patients. The potential for transfer of MDROs to dental office staff from a patient, to a patient from a dental health care provider, or from the dental practice environment to patient or health care provider has not yet been established. But as much as 25% of health care workers may carry MRSA in their nasal passages.1,7,8
SOURCES OF CONTAMINATION IN A DENTAL OFFICE
Most sterilization and cross-contamination procedures in dental practices are focused on eliminating the transfer of microbes from patient to patient or between office staff and patient. However, contamination can occur from inanimate surfaces to patient or practitioner. Thus, the careful sterilization of instruments and trays; cleaning and disinfection of dental chairs and cabinets; and placing disposable covers over light handles, radiograph controls, handpieces, three-way syringes, and head rests are important contamination control practices in dental settings.10
 Often overlooked as pathogen reservoirs are computer keyboards, computer mouse devices, telephones, pens, patient charts, ultrasonic controls, light cure wands, front desk counter tops, stethoscopes, blood pressure cuffs, door knobs, and wash basins in restrooms.12-16 Since computers are used by most dental practices in the United States, office computers and their ancillary components need to be examined as possible contaminants in the fight against MDROs. The computer keyboard/ mouse is found in the operatory, at the receptionist’s desk, the business office, the dentist’s desk, and occasionally, the sterilization station and laboratory. The same computer station may be used by several individuals during the day—opening up the possibility for crosscontamination. To investigate this possibility further, we conducted a small research study in 2008 at Skagit Valley Hospital in Mount Vernon, Wash, and 12 dental practices within the local community. The micro-assays were done in Seattle at a commercial laboratory.
Often overlooked as pathogen reservoirs are computer keyboards, computer mouse devices, telephones, pens, patient charts, ultrasonic controls, light cure wands, front desk counter tops, stethoscopes, blood pressure cuffs, door knobs, and wash basins in restrooms.12-16 Since computers are used by most dental practices in the United States, office computers and their ancillary components need to be examined as possible contaminants in the fight against MDROs. The computer keyboard/ mouse is found in the operatory, at the receptionist’s desk, the business office, the dentist’s desk, and occasionally, the sterilization station and laboratory. The same computer station may be used by several individuals during the day—opening up the possibility for crosscontamination. To investigate this possibility further, we conducted a small research study in 2008 at Skagit Valley Hospital in Mount Vernon, Wash, and 12 dental practices within the local community. The micro-assays were done in Seattle at a commercial laboratory.
THE STUDY
The purpose of this study was to illustrate that there are contaminated surfaces within the dental office that clinicians may not consider as reservoirs of MDROs, that these surfaces can be somewhat effectively cleansed, and that washing hands frequently is a recommended method of avoiding the spread and acquisition of these organisms.
Although there are many other surfaces within a dental office that may have the same potential for cross-contamination, this study examined only the computer keyboard/ mouse. To evaluate the initial level of contamination of keyboards/mouse devices, 88 computer keyboards/mouse devices, which had never been cleansed or disinfected, were sampled for microbes present. Then three groups were assembled. The first consisted of 13 conventional keyboards/mouse devices that were not disinfected; the second contained 14 conventional keyboards/ mouse devices that were disinfected/ cleansed three times a day with a disinfectant; and the third encompassed 12 sealed keyboard/mouse devices that were disinfected/ cleansed three times a day. All groups were evaluated for contamination over a period of 4 months. These computer keyboard/ mouse devices were located in various locations in dental offices— reception, operatories, and the dentist’s desk. The phones, desk, and pens at the work stations in the second and third groups were disinfected three times a day as well.
A disinfectant wipe consisting of 12% n-alkyl (60% C14, 30% C16, 5% C12, 5% C18) dimethyl benzyl ammonium chloride; n-alkyl (68% C12, 32% C14) dimethyl ethyl benzyl ammonium chloride; and 63.25% isopropyl alcohol was used for 2 minutes and refreshed if it became dry. This commercial disinfectant method was selected as representative of one typically used in dental practices. In-service training was provided for all staff who might come in contact with these keyboards/mouse sites.
The sealed keyboard/mouse was flat and impervious to fluids. A flashing light reminded the user to disinfect the keyboard and mouse at a preset interval.
To determine the level of microbial contamination, a single sterile swab moistened with Trypticase soy broth (TSB) was wiped over the entire keyboard or mouse surface. The swab was placed in 2 ml of TSB and immediately transported to the laboratory. Samples were taken at random, unannounced intervals over 3 months.
RESULTS
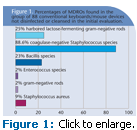
 These data are reported as a percent of total keyboards/mouse sites examined with the specific organism detected. Of the 88 conventional keyboards/mouse devices not disinfected or cleansed in the initial evaluation, 25% harbored lactose-fermenting gram-negative rods, 88.6% coagulase-negative Staphylococcus species, 23% Bacillis species, 2% Enterococcus species, 2% gram-negative rods, and 9% Staphylococcus aureus (see Figure 1).
These data are reported as a percent of total keyboards/mouse sites examined with the specific organism detected. Of the 88 conventional keyboards/mouse devices not disinfected or cleansed in the initial evaluation, 25% harbored lactose-fermenting gram-negative rods, 88.6% coagulase-negative Staphylococcus species, 23% Bacillis species, 2% Enterococcus species, 2% gram-negative rods, and 9% Staphylococcus aureus (see Figure 1).
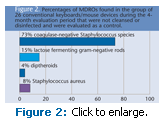
In the 4-month evaluation period, 26 conventional keyboards/mouse devices not cleansed or disinfected were evaluated as a control. This group harbored 73% coagulase-negative Staphylococcus species,15% lactose fermenting gram-negative rods, 4% diptheroids, and 8% Staphylococcus aureus (Figure 2). These data are similar to findings in the not cleansed/ disinfected group in the previous paragraph.
Ten sealed keyboards/mouse pairs wiped three times a day with disinfectant harbored 5% coagulase-negative Staphylococcus species, 10% Bacillus species, and 10% lactose fermenting gram-negative rods. Three sealed disinfected/ cleansed keyboard/mouse devices had no detectable organisms (Figure 3).
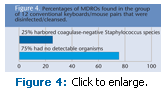
Of the 12 conventional keyboards/ mouse pairs that were disinfected/cleansed, 25% harbored coagulase-negative Staphylococcus species; and 75% had no detectable organisms (Figure 4). Both of these later groups had fewer detectable organisms than either of the keyboard/ mouse groups that were not cleansed/ disinfected.
DISCUSSION
1. Observations21,22 in the medical literature that clinic and hospital computer keyboards harbor bacteria related to MDROs, such as Staphylococcus aureus, enterococci, and Gram-negative bacilli, were also true for the dental office.
2. Both conventional and sealed keyboard/ mouse devices experienced a reduction in detectable organisms when disinfected three times a day. Although disinfected keyboard/ mouse pairs had reduced levels, the potential MDROs enterococci and Staphylococcus aureus were still found.
3. Using disinfectant wipes on a conventional computer keyboard did no apparent damage to the keyboard.
4. With respect to disinfection/cleansing, a sealed keyboard/mouse may offer some advantages over a conventional keyboard/ mouse, including the ease of wiping, compliance, and the light reminding the operator to disinfect at regular intervals.
5. The data suggest cleansing the work area with disinfectant wipes three times a day (keyboard, mouse, desktop, pens, and telephone) can reduce detectable organisms.
STUDY WEAKNESSES
This trial did not evaluate the survival time of pathogens on surfaces in dental practices nor did it determine the optimal interval for disinfection of these surfaces. The rate of re-infection of a disinfected surface may vary from site to site as will the effectiveness of the disinfection effort. This study did not evaluate whether the species related to MDROs were resistant to a broad spectrum of antibiotics. The intent of the study was to demonstrate the potential for these organisms to exist on surfaces within a dental practice.
COMPLIANCE
Compliance with standard infection control methods (disinfecting on a regular basis, washing hands, avoiding potential contamination from vectors [organisms that transmit pathogens], not having food/ coffee at the desk, leaving treatment areas with gloves on or using a gloved hand on a keyboard/mouse, chart, or radiographs) and a disinterest by some nonclinicians in complying with the protocol were the main difficulties in this study. This failure to comply with the study protocol could account for the contamination of some of the cleansed keyboards/ mouse devices but it does not invalidate the results reported. On the contrary, this failure to comply with standard cross-contamination procedures underscores the realities of dental practices and why all staff need to be aware of contact with potentially contaminated surfaces. In-service training and reinforcement of disinfection standards may be helpful in reducing the level of pathogens in environmental reservoirs and in eliminating crosscontamination in the dental office. But in-service training intrinsically does not eliminate the necessity for caution. Although careful attention was given to in-service training of nonclinical personal in this trial our observations illustrate the all-toocommon failure to adhere to cross-contamination guidelines.
Patient charts and radiographs are vectors that cannot be disinfected. Although charts and radiographs should not be in treatment areas, the fact is they often are. The reality in many practices is that charts and radiographs are occasionally touched with the gloved hand, exposed to aerosol from handpieces, passed to the front desk, and taken from room to room and to the dentist’s desk. Certainly they find their way several times a day to a keyboard where a staff member can enter data from the chart. After disinfection of keyboards, mouse devices, phones, desktops, and pens, the staff member who enters the data at the computer may immediately contaminate that station. For example, he or she may receive a chart from a clinician and enter data in the computer, take money or checks from patients, answer the telephone, or go to a station that may not have been disinfected, such as the copy machine.
Other areas of possible contamination are gowns and clothing, blood pressure cuffs, hand-held computer devices, cell phones, and pens. Infection from cross-contamination of these sites to the patient or from the patient to these sites may occur if basic contamination containment practices are not followed by all members of the dental health care team— regardless of whether their duties are clinical or office-related.
Clinicians and staff are generally aware that they have a risk of contracting infections from patients when they are not protected with barriers such as masks, gloves, full shield eye protection, and gowns. Barriers such as gowns, gloves, masks, and eye/face protection should be sufficient to protect patients and dental health care providers chairside regardless of the level of contamination of surfaces outside the operatory. However, since it is virtually impossible outside the operatory to avoid coming in contact with organisms on patient charts and radiographs, front desk surfaces, telephones, pens, computers, etc, dental staff need to take precautions. It may sound simplistic but the single most effective recommendation for preventing contamination is to wash hands frequently throughout the day, before and after gloving, before eating, and before leaving the dental practice environment.10,15,19,20 The Centers for Disease Control and Prevention recommendations state: “In health care settings, handwashing can prevent potentially fatal infections from spreading from patient to patient and from patient to health care worker and vice-versa. The basic rule in the hospital is to cleanse hands before and after each patient contact by either washing hands or using an alcohol-based hand rub.”19,20
Recognition of potential reservoirs of bacterial contamination within the dental office, following basic hygiene precautions such as washing hands, and the use of barrier procedures chairside can reduce the risk of MDRO transmission to dental patients and dental health care providers.
REFERENCES
- Healthcare-Associated Methicillin Resistant Staphylococcus aureus (HA-MRSA). Available at: www.cdc.gov/ncidod/dhqp/ar_MRSA.html. Accessed May 12, 2009.
- Herold B, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593-598.
- Smith TL, Pearson ML, Wilcox KR, et al. Emergence of vancomycin resistance in Staphylococcus aureus. N Engl J Med. 1999;340:493-501.
- Rotun SS, McMath V, Schoonamker DJ, et al. Staphylococcus aureus with reduced susceptibility to vancomycin isolated from a patient with fatal bacteremia. Emerg Infect Dis. 1999;5:147-149.
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178-182.
- Moellering RC Jr. The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann Intern Med. 2006;144:368-370.
- Graham PL 3rd, Lin SX, Larson EL. A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med. 2006;144:318-325.
- Changing pattern of community-acquired skin and soft-tissue infection with antibiotic-resistant Staphylococcus aureus. Ann Intern Med. 2006;144:309-317.
- Zeller JL, Burke AE, Glass RM. MRSA infections. JAMA. 2007;17;298:1826.
- Infection Control and Guidelines and Recommendations. Recommended Infection Control Practices for Dentistry. Available at: www.cdc.gov/OralHealth/infectioncontrol/guidelines. Accessed May 12, 2009.
- Rice EW, Rich WK, Johnson CH, Lye D. The role of flushing dental water lines for the removal of microbial contaminants. Public Health Rep. 2006;121:270–274.
- Walker JT, Bradshaw DJ, Bennett AM, Fulford MR, Martin MV, Marsh PD. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol. 2000;66:3363-3367.
- Shearer BG. Biofilm and the dental office. J Am Dent Assoc. 1996;127:181-189.
- Mills S. Waterborne pathogens and dental waterlines. Dent Clin North Am. 2003;47:545-557.
- Machado-Carvalhais HP, Ramos-Jorge ML, Auad SM, Martins LH, Paiva SM, Pordeus IA. Occupational exposure to potentially infectious biological material in a dental teaching environment. J Dent Educ. 200;72:1201-120.
- Kenyon BJ, Van Zyl I, Louie KG. Comparison of cavity preparation quality using an electric motor handpiece and an air turbine dental handpiece. J Am Dent Assoc. 2005;136:1101-1105.
- Centers for Disease Control and Prevention (CDC). Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006-January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:325-329.
- Community-Associated Methicillin Resistant Staphylococcus aureus (CA-MRSA) Available at: www.cdc.gov/ncidod/dhqp/ar_mrsa_ca.html. Accessed May 12, 2009.
- Guideline for Hand Hygiene in Healthcare Settings—2002. Available at: www.cdc.gov/handhygiene. Accessed May 12, 2009.
- Guideline for Hand Hygiene in Health-Care Settings. Available at: www.cdc.gov/mmwr/PDF/rr/rr5116.pdf. Accessed May 12, 2009.
- Rutala WA, White MS, Gergen MF, Weber DJ. Bacterial contamination of keyboards: efficacy and functional impact of disinfectants. Infect Control Hosp Epidemiol. 2006;27:372-377.
- Weber DJ, WA Rutala. The emerging infectious pathogens Cryptosporidium, E. coli O157:H7, Helicobacter pylori, and Hepatitis C: Epidemi ology, environmental survival, efficacy of disinfection and control measures. Infect Control Hosp Epidemiol. 2001;22:306-315.
From Dimensions of Dental Hygiene. June 2009; 7(6): 42-45.