
Beyond the Probe
New technologies and developments in periodontal assessment and diagnosis.
The understanding of the etiology and pathogenesis of periodontal diseases has undergone significant changes in the recent past. As a result, the diagnostic tools used to accurately assess disease have required modifications as well. Clinicians face a critical and very difficult decision—determining if a patient’s periodontal disease is active, arrested, or in remission. These diagnostic tools are designed to help clinicians differentiate between gingivitis and periodontitis.
Although our current evidence-based concepts in periodontology are evolving, we still use conventional clinical assessments.1 When making a periodontal diagnosis, clinicians consider: the presence or absence of clinical detectable inflammation; the extent and pattern of clinical attachment loss; patient’s age; rate of progression; and presence or absence of miscellaneous signs and symptoms including pain, ulceration, and the amount of observable plaque and calculus.2 However, research shows that most of these traditional diagnostic means have good sensitivity but only fair specificity in diagnosing sites and/or patients with active disease progression.3 Thus, the development of new diagnostic tools are focused on a patient’s particular periodontal disease and response to treatment.
Today, we discuss the risk profile with patients and talk about risk factors that might predispose them to disease initiation and progression.4 New developments are available that improve our diagnostic power. Improved diagnostics should: differentiate between periodontal diseases, identify disease initiation and progression, identify persons and teeth that are susceptible to disease initiation and progression, and monitor the response to treatment.5
Digital Radiography
Digital imaging has been on the market since 1987 and the evolution of its technology has made it a viable alternative to film-based imaging. Some benefits of digital imaging include the elimination of chemical processing and shorter exposure-to-display time. Furthermore, it can be integrated with existing electronic office and patient-management systems. Currently, two different technologies for digital imaging are available: solid-state detectors and photostimulable phosphor.
The solid-state detectors are based either on charge-coupled device (CCD) or complementary metal-oxide device (CCD) or complementary metal-oxide semiconductor (CMOS) technology. One great advantage of this system is that the images are immediately available to the radiographer. However, the sensors’ rigidity and cable attachment can present a challenge in sensor placement and patient management.
Digital imaging systems based on photostimulable phosphor (PSP) are handled similarly to film imaging. The exposed plates are scanned in an external laser scanner that generates the digital image data for storage and display on the computer. The plates can then be erased and reused.
Digital imaging does not improve diagnostic efficacy per se. Film technologies still remain equivalent to digital receptors. However, the advancements in digital imaging allowed the development of other promising technologies such as digital subtraction radiography (DSR) and tuned aperture computed tomography (TACT® ).
DIGITAL SUBTRACTION RADIOGRAPHY
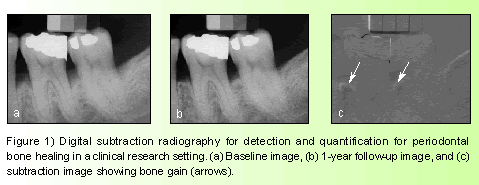
As early as 1935, Ziedes des Plantes described subtraction imaging, applying it on conventional film imaging. The later development of microcomputers facilitated the digitizing and subtraction of conventional radiographs.6 In the digital subtraction process, two images of the same object are registered and the image intensities of corresponding pixels are subtracted, then a uniform difference image is produced. If a change in the follow-up image has occurred, the change shows up as a brighter area when it represents gain and as a darker area when it represents loss (Figure 1).7
Several studies have demonstrated the high diagnostic utility of DSR compared to conventional radiography.8 Subtraction images allow detection of mineral change of as little as 5%. The current DSR research concentrates on developing image processing techniques to facilitate image standardization and refining image analysis techniques to detect and quantify osseous changes. However, clinical application of DSR must be improved to become a routine procedure.
TUNED APERTURE COMPUTED TOMOGRAPHY
TACT® was developed with the focus on providing a three-dimensional radiographic assessment of dentoalveolar tissues. This technology was developed to keep both the financial cost and the dose low for patients.
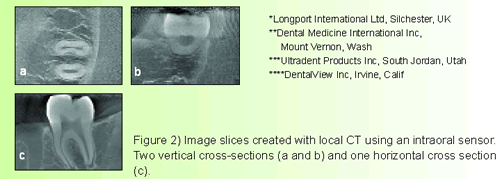
This technique uses basic concepts of tomosynthesis—by shifting and combining a set of basis projections, arbitrary slices through the object can be brought into focus.9 The basis projections are conventional transmission radiographs. Each radiograph is taken from a different angle relative to the object and the receptor. Each slice is a two-dimensional representation of the object at a different location in the third dimension (Figure 2). 10
In a number of studies, TACT and TACT subtraction have shown promise in the detection and localization of osseous changes in the crestal bone.11 The ability to detect osseous defects around dental implants also showed improvement in the studies.12
Another promising technology is the local computed tomography (LCT) that generates image details in three-dimensions at reduced patient dose and cost. This modality of CT appears to be helpful in assessments of alveolar bone loss and prospective implant sites.
ANALYSIS OF GINGIVAL CREVICULAR FLUID
More than 65 gingival crevicular fluid (GCF) components have been examined as possible markers for the progression of periodontitis: host-derived enzymes and their inhibitors, inflammatory mediators and host-response modifiers, and tissue-breakdown products.13
The problem is that clear distinctions between periodontitis and gingivitis sites as well as between progressing and nonprogressing sites do not exist. This overlap between inflamed progressing and nonprogressing sites often presents false positive test results.14 The same problem of false positive test results occurs in the analyses of the inflammatory mediators and host response modifiers.
Promising results have been seen in analyses of host breakdown products as potential GCF markers of disease progression. Chondroitin-4-sulfate (C-4-S), a bone-specific glycosaminoglycan, is about 94% of the glycosoaminoglycans content of alveolar bone.15 Studies have shown a statistically significant correlation between GCF content of C-4-S and probing depth and attachment loss.16 Yet, a lack of longitudinal studies assessing the diagnostic value of C-4-S still exists.
Pyridinoline crosslinks of carboxyterminal telopeptide to type I collagen (ICTP) is a good marker of bone collagen degradation.17 Some preliminary studies have evaluated this bone degradation product as a potential marker for progression of periodontitis.18-20 ICTP was found at higher concentrations in deeper pockets than in more shallow pockets. However, scaling and root planing (SRP) was not able to reduce the GCF levels of ICTP at up to 6 months post-treatment.21
Periodontal Ultrasonography
Some early research has examined the use of ultrasonography in periodontology as a means of noninvasive diagnostics.22 The main components of ultrasonography equipment are the transducer, pulser, receiver, memory, monitor, and keyboard. The transducer converts the electronic impulse (arising from the pulser) into ultrasound waves. It also converts the returning ultrasound echoes (from the tissues) into an electronic impulse to produce an image. The ultrasound frequencies used in diagnosis range between 1 MHz and 20 MHz. An ultrasonic beam entering tissues is either absorbed, reflected, refracted, or scattered. The reflected portion is received by the machine and used for reconstruction of the ultrasonic image.23
A recent study using the ULTRADERM ultrasonic scanner* that works at the frequency of 20 MHz in an animal model has shown that periodontal ultrasonography can produce images suitable for the assessment of the periodontium as well as accurate measurement of the dimensional relationship between hard and soft structures.23
The technique of ultrasonography has also been applied in human subjects. In different studies, the device was used to evaluate gingival thickness before and after mucogingival therapy for root coverage.24 It was also used to assess the dynamics of mucosal dimensions after root coverage with connective tissue grafts25 or bioresorbable barrier membranes.26
Future research will focus on optimizing the image quality, especially for the soft tissue structures. Also changes in the equipment (higher frequencies, different focal lengths) could result in better image resolution.
PREVISER RISK ASSESSMENT
PreViser’s Oral Health Information Suite™** (previously called the RiskCalculator™) is a computer- and web-based risk calculator for periodontal patients, also called a Periodontal Risk Calculator (PRC). The PRC calculates the risk for initiation of periodontal disease in healthy patients and the risk of disease progression in already affected subjects. The system evaluates certain factors as potential risk factors for disease. A risk factor is defined as part of the causal chain of disease or exposure to the causal chain, which, if present, directly increases the probability of disease occurring and, if absent, reduces the probability.
The calculation of risk involves mathematical algorithms that use nine factors including: patient’s age, smoking history, diagnosis of diabetes, history of periodontal surgery, pocket depths, furcation involvements, restorations or calculus below the gingival margin, radiographic bone height, and vertical bone lesions. Its predictive algorithm was validated using an existing database of periodontal patients.27,28 All these determinants factor into a risk score on a scale of one to five for periodontal deterioration for each individual.
The use of PRC in the practice setting and suggested treatment options over time could lead to more uniform decision-making about periodontitis. If long-term studies support the promising initial results of PRC’s accuracy, this tool could have positive influence on the reduction in disease incidence, improved oral health, reduction in extensive treatment needs, and reduction in cost of care.29,30
DETEC TAR PROBE
The DetecTar Probe*** is a diagnostic device that aids the practitioner in detecting subgingival calculus efficiently. The manufacturer states the device is able to identify subgingival calculus with an efficacy of about 91% in periodontal pockets up to 10 mm in depth. Its technology is based on light emitting diodes (LEDs) at extremely narrow wavelength bands (20-40 nanometers). An LED light emits from the tip of the probe and is returned off the root surface. This light is sensored by a fiber optic lead and converted into an electrical signal for further analysis. A computer-processed algorithm determines if the probe is in actual contact with calculus and activates an auditory and green light signal to indicate the presence of calculus. The DetecTar probe could be a promising device for the patient to experience the presence of subgingival calculus via hearing and seeing and it could help the clinician identify residual subgingival calculus during the nonsurgical treatment phase.
PERIODONTAL ENDOSCOPE
One major problem during the instrumentation of a periodontal pocket is the lack of visualization. The dental endoscope**** is designed to enable visualization of the gingival sulcus, providing the precise location of the biofilm, root deposits, granulation tissue, caries, and root fractures.31,32 A disposable sterile sheath is placed around the endoscope that is then attached to an explorer-probe guiding the endoscope subgingivally. An attached soft tissue shield helps retract the gingiva to visualize the root surface directly. The image of the sulcus content is displayed on a flat screen monitor with a magnification of 22x to 48x. Perioscopy is a case- and site-specific technique that is most effective after initial therapy because sites with severe inflammation are usually difficult to view due to excessive hemorrhage and granulation tissue.
![]() Figure 3) Para Audio-Probe. The handpiece is made of aluminum, the probe of stainles steel.
Figure 3) Para Audio-Probe. The handpiece is made of aluminum, the probe of stainles steel.
AUTOMATED AND VOICE-ACTIVATED PERIODONTAL PROBING SYSTEMS
The Florida Probe®* is a constant force electronic probe connected to a computer for instantaneous data capture and storage. Probing forces are preset at 0.2 N. Once the probe is in place via a foot pedal the computer automatically captures the measurements to the nearest 0.1 mm.33
Another automated probe currently on the market is the Paro Audio-Probe** (Figure 3). This probe ensures an optimal and maintained probing forces of 0.25 N (Newton) that is controlled with an acoustic signal. The probe tip has a point diameter of 0.45 mm. The measurement scale of the probe corresponds to the marking of the Michigan probe.34
The voice-activated periodontal probe market is growing. Dentrix Voice®*** uses a microphone/headset for the recording of periodontal and restorative chartings. The company claims that, within 5 minutes, a unique user profile can be created with an over 90% accuracy rate. This dictation technology can be integrated into the DENTRIX computer software through Microsoft Windows® .
The STM® Probe**** offers voice response of probing depths and bleeding points with a pressure sensitive handpiece. The system operates independently of other computer hardware and prints out a record of the bleeding points and probing depths. The unit audibly announces probe measurements and bleeding points so patients can hear them.
The PerioPal® System***** uses a different approach for recording the periodontal examination. Instead of using dictation technologies, PerioPal® uses a voice command system so that only commands available for input are recognized. The company claims that by using these specific commands the word recognition is greatly increased. The headset and specific microphone for this system are attached to either a laptop or desktop that uses Windows XP as the operating system.
As we learn more about periodontal disease and its relationship to overall health, our diagnostic tools must evolve as well. Clinicians must also weigh the evidence when evaluating new products to make the most efficacious decisions for their practices and their patients.
*Florida Probe Corp Gainsville, Fla
**Heico Dent, Oberegg, Switzerland
***Dentrix, American Fork, Utah
****Pro-Dentec® , Batesville, Ark
*****PerioPal, Beaumont, Tex
REFERENCES
- Armitage GC. Clinical evaluation of periodontal diseases. Periodontol 2000. 1995;7:39-53.
- Caton J. Periodontal diagnosis and diagnostic aids. In: Nevins M, Becker W, Kornman K, eds. Proceedings of the World Workshop in Clinical Periodontics July 23-27 1989. Chicago: American Academy of Periodontology; 1989 .
- Haffajee AD, Socransky SS, Goodson JM. Clinical parameters as predictors of destructive periodontal disease activity. J Clin Periodontol . 1983;10:257-265.
- Beck JD. Issues in assessment of diagnostic tests and risk for periodontal diseases. Periodontol 2000 . 1995;7:100-108.
- Williams RC. Periodontal disease activity: Current concepts. In: Wilson TG Jr, Kornman KS, Newman MG, eds. Advances in Periodontology . Tokyo: Quintessence; 1992:58-73.
- Grondahl HG, Grondahl K, Webber RL. A digital subtraction technique for dental radiography. Oral Surg Oral Med Oral Pathol . 1983;55:96-102.
- Mol A. Imaging methods in periodontology. Periodontol 2000 . 2004;34:34-48.
- Reddy MS, Jeffcoat MK. Methods of assessing periodontal regeneration. Periodontol 2000 . 1999;19:87-103.
- Groenhuis RA, Webber RL, Ruttimann UE. Computerized tomosynthesis of dental tissues. Oral Surg Oral Med Oral Pathol . 1983;56:206-214.
- Webber RL, Horton RA, Tyndall DA, Ludlow JB. Tuned-aperture computed tomography (TACT). Theory and application for three-dimensional dento-alveolar imaging. Dentomaxillofac Radiol . 1997;26:53-62.
- Horton RA, Ludlow JB, Webber RL, Gates W, Nason RH Jr, Reboussin D. Detection of peri-implant bone changes with axial tomosynthesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 1996;81:124-129.
- Webber RL, Horton RA, Underhill TE, Ludlow JB, Tyndall DA. Comparison of film, direct digital, and tuned-aperture computed tomography images to identify the location of crestal defects around endosseous titanium implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 1996;81:480-490.
- Armitage GC. Analysis of gingival crevice fluid and risk of progression of periodontitis. Periodontol 2000 . 2004;34:109-119.
- Kennett CN, Cox SW, Eley BM. Investigations into the cellular contribution to host tissue proteases and inhibitors in gingival crevicular fluid. J Clin Periodontol . 1997;24:424-431.
- Waddington RJ, Embery G, Last KS. Glycosaminoglycans of human alveolar bone. Arch Oral Biol . 1989;34:587-589.
- Smith AJ, Addy M, Embery G. Gingival crevicular fluid glycosaminoglycan levels in patients with chronic adult periodontitis. J Clin Periodontol . 1995;22:355-361.
- Risteli J, Elomaa I, Niemi S, Novamo A, Risteli L. Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clin Chem . 1993;39:635-640.
- Oringer RJ, Palys MD, Iranmanesh A, et al. C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clin Oral Implants Res . 1998;9:365-373.
- Palys MD, Haffajee AD, Socransky SS, Giannobile WW. Relationship between C-telopeptide pyridinoline cross-links (ICTP) and putative periodontal pathogens in periodontitis. J Clin Periodontol . 1998;25:865-871.
- Giannobile WV. C-telopeptide pyridinoline cross-links. Sensitive indicators of periodontal tissue destruction. Ann N Y Acad Sci . 1999;878:404-412.
- Al-Shammari KF, Giannobile WV, Aldredge WA, et al. Effect of non-surgical periodontal therapy on C-telopeptide pyridinoline cross-links (ICTP) and interleukin-1 levels. J Periodontol . 2001;72:1045-1051.
- Spranger H. Ultrasonic diagnosis of marginal periodontal diseases. Int Dent J . 1971;21:442-455.
- Tsiolis FI, Needleman IG, Griffiths GS. Periodontal ultrasonography. J Clin Periodontol . 2003;30:849-854.
- Muller HP, Eger T, Schorb A. Gingival dimensions after root coverage with free connective tissue grafts. J Clin Periodontol . 1998;25:424-430.
- Muller HP, Stahl M, Eger T. Root coverage employing an envelope technique or guided tissue regeneration with a bioabsorbable membrane. J Periodontol . 1999;70:743-751.
- Muller HP, Stahl M, Eger T. Dynamics of mucosal dimensions after root coverage with a bioresorbable membrane. J Clin Periodontol . 2000;27:1-8.
- Page RC, Krall EA, Martin J, Mancel L, Garcia RI. Validitiy and accuracy of a risk calculator in predicting periodontal disease. J Am Dent Assoc . 2002;133:569-576.
- Page RC, Martin J, Krall EA, Mancl L, Garcia R. Longitudinal validation of a risk calculator for periodontal disease. J Clin Periodontol . 2003;30:819-827.
- Axelsson P, Lindhe J, Nystrom B. On the prevention of caries and periodontal disease. Results of a 15-year longitudinal study in adults. J Clin Periodontol . 1991;18:182-189.
- Fors UG, Sandberg HC. Computer-aided risk management—a software tool for the Hidep model. Quintessence Int . 2001;32:309-320.
- Stambaugh RV. A clinician’s 3-year experience with perioscopy. Compend Contin Educ Dent . 2002;23:1061-1070.
- Stambaugh RV, Myers G, Ebling W, Beckman B, Stambaugh K. Endoscopic visualization of the submarginal gingiva dental sulcus and tooth root surfaces. J Periodontol . 2002;73:374-382.
- Araujo MW, Hovey KM, , Benedek JR, et al. Reproducibility of probing depth measurement using a constant-force electronic probe: analysis of inter- and intraexaminer variability. J Periodontol . 2003;74:1736-1740.
- Mombelli A, Muhle T, Frigg R. Depth-force patterns of periodontal probing. Attachment-gain in relation to probing force. J Clin Periodontol . 1992;19:295-300.
From Dimensions of Dental Hygiene. January 2005;3(1):10-12, 14.

