 SomeMeans /iStock / Getty Images Plus
SomeMeans /iStock / Getty Images Plus
Treating COVID-19
| Jessica Fagan, RDH, BS, MA—a full-time faculty member at Carrington College in Sacramento, California—is blogging for Dimensions of Dental Hygiene about COVID-19. |
Physicians and other healthcare providers, scientists, and researchers are all working tirelessly to combat the COVID-19 outbreak. While some are testing the efficacy of existing medications, others are assessing the potential of new treatment options. And the big question is: When will we see a vaccine?
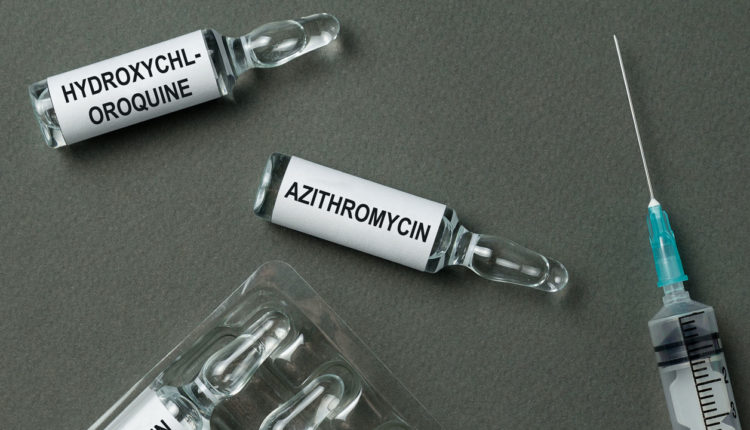
Currently, there are no drugs or other therapies approved by the United States Food and Drug Administration (FDA) for the treatment of COVID-19. However, medications, such as remdesivir and hydroxychloroquine/chloroquine, are being tested for their efficacy in combatting this virus. Remdesivir is an antiviral, intravenous drug that has shown to be effective against other strains of the coronavirus.1 It is still too early to tell how effective this drug will be against COVID-19, but numerous clinical trials have begun to evaluate its success. Hydroxychloroquine and chloroquine are oral prescription drugs that have been used in the past to treat malaria. Normally, using such drugs outside of their scope would not be an option. However, recently the FDA issued an Emergency Use Authorization for emergency use of these drugs for the treatment of COVID-19. Find out more here: https://www.fda.gov/media/136534/download
In one small initial study, 100% of the patients treated with both hydroxychloroquine and azithromycin showed negative nasopharyngeal samples for viral load by day six.2 These results are hopeful, but it is still too early to determine if these effects have any clinical significance when used on a larger sample size.
Another treatment option recently approved by the FDA for testing is the use of convalescent plasma therapy, also referred to as passive antibody therapy. This therapy takes the antibodies from an individual who has healed from the virus and transfuse them into someone who is still sick with the virus. The hope is that the sick individual’s immune system gets a boost from the infusion of antibodies. This type of treatment has been beneficial among those infected with other coronavirus strains, such as severe acute respiratory syndrome, in the past.3 Recent clinical trials have begun to test the use of this treatment option on patients with COVID-19. In one study, 10 patients with severe symptoms were given the convalescent plasma and within three days of administration, clinical symptoms were significantly improved. Additionally, the viral load was undetectable in seven out of 10 of these patients.4 These studies are still in very early stages and will need further research.
The true end goal, however, would be the manufacture of a vaccine against future outbreaks. Many companies and academic institutions are in a race to create this vaccine. Moderna Therapeutics, a biotech company, was the first to ship a potential vaccine to the National Institutes of Allergy and Infectious Diseases (NIAID),5 and on March 16, the first doses of the vaccine were administered to a group of volunteers participating in a small clinical trial.6 While this is promising news, experts still stress that it could be quite awhile before any vaccine becomes widely available. Anthony Fauci, MD, director of the NIAID, recently expressed that 12 months to 18 months is a realistic timeline for vaccine availability. Only time will tell.
References
- US Centers for Disease Control and Prevention. Coronavirus Disease 2019 (Covid-19) Therapeutic Options. https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7102549/
- Chen L, Xiong J, Shi Y. Convalescent plasma as a potential therapy for COVID-19. The Lancet Infectious Diseases. 2020;20. Available at: https://www.thelancet.com/pdfs/journals/laninf/PIIS1473-3099(20)30141-9.pdf
- Duan K, Liu B, Li C, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. MedRxiv. 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.03.16.20036145v1
- Park A. COVID-19 Vaccine Shipped, and Drug Trials Start. Time. 2020. Available at: https://time.com/5790545/first-covid-19-vaccine/
- Cohen J. Vaccine designers take first shots at COVID-19. Science. 2020;368:14-16. Available at: https://science.sciencemag.org/content/368/6486/14/tab-pdf

