The Role of Probing and Material Choice in Implant Health
Monitoring implant health requires precise diagnostic techniques, with probing recognized as a key assessment tool. However, the choice between metal and plastic probes remains controversial, as both present unique risks to implant surfaces and peri-implant tissues.
Effective long-term implant health monitoring is crucial following the placement of a dental implant. Practitioners must possess a comprehensive understanding of how monitoring is used to direct the appropriate maintenance care.1
Various clinical parameters are used to assess implant health, including mobility, radiography, gingival alterations, pain, occlusion, probing pocket depth, and bleeding on probing.2-6 Probing around dental implants has been a subject of debate in the past, however, it is now widely recognized as one of the most valuable diagnostic tests for evaluating implant health. Clinically, bleeding on probing and probing depth measurements are the most beneficial when baseline measurements are used for comparison.3,7-10
Probing around and adjacent to an implant will cause some frictional wear or tribological interaction between the probe and the implant and/or abutment material.11 The science of tribology and frictional wear is complex and depends on factors such as loading forces, surface texture, rate of movement, hardness of the material, adhesion, lubrication and other factors.11,12 Thus, the probing force and materials are important as is the environment.
Periodontal probes are made from either metal or plastic, and the choice between the two types continues to be a topic of debate.13-17 Metal instruments, including titanium, have been criticized for producing scratches on implants and abutments, while plastic instruments have been associated with leaving debris deposits on dental implants and implant components.13-17
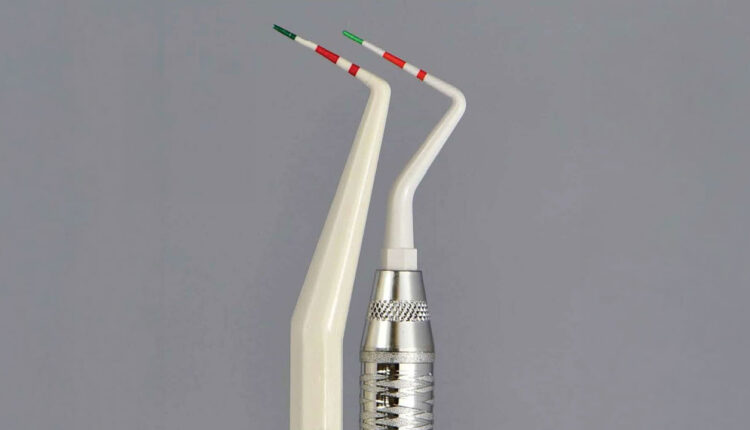
Some plastic probes have colored, graduated depth markings (green and red) to aid in visualizing and measuring (Figure 1). The instructions for use indicate that the probes can be utilized up to 30 times and should be replaced when the colored markings become poorly visible or are removed. However, it is unclear how these color markings degrade — whether it’s during the sterilization process or through use. The biocompatibility of the paint is not disclosed other than it is a food grade-material registered with the United States Food and Drug Administration. If the color comes off as the probe moves against the implant or abutment surface, the resulting contamination may impact the peri-implant tissues.18-20
References
- National Institutes of Health consensus development conference statement: dental implants. J Am Dent Assoc. 1988;117:509-513.
- Bauman GR, Mills M, Rapley JW, Hallmon WH. Clinical parameters of evaluation during implant maintenance. Int J Oral Maxillofac Implants. 1992;7:220-227.
- Coli P, Christiaens V, Sennerby L, De Bruynet H. Reliability of periodontal diagnostic tools for monitoring peri-implant health and disease. Periodontol 2000. 2017;73:203-217.
- Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur J Oral Sci. 1998;106:527-551.
- Merli M, Bernardelli F, Giulianelli E, Toselli I, Moscatelli M. Inter-rater agreement in the diagnosis of mucositis and peri-implantitis. J Clin Periodontol. 2014;41:927-933.
- Misch CE, Perel ML, Wang HL, Sammartino G, Galindo-Moreno P. Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008;17:5-15.
- Mombelli A, Lang NP. Clinical parameters for the evaluation of dental implants. Periodontol 2000. 1994:4:81-86.
- Luterbacher S, Mayfield L, Brägger U, Lang NP. Diagnostic characteristics of clinical and microbiological tests for monitoring periodontal and peri-implant mucosal tissue conditions during supportive periodontal therapy (SPT). Clin Oral Implants Res. 2000;11:521-529.
- Renfert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucsositis, peri-implantitis: case definitions and diagnostic considerations. J Periodontol. 2018;89:S304.
- Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89:S313-S318.
- Dowson D. Bio-tribology. Faraday Discuss. 2012;156:9-30.
- Cha J, Wadhwani C, Wang M, Hokett SD, Katancik J. Instrument selection and application used to probe dental implants. Int J Oral Maxillofac Implants. 2019;34:115-123.
- Agar JR, Cameron SM, Hughbanks JC, Parker MH. Cement removal from restorations luted to titanium abutments with simulated subgingival margins. J Prosthet Dent. 1997;78:43-47.
- Mengel R, Buns CE, Mengel C, Flores-de-Jacoby L. An in vitro study of the treatment of implant surfaces with different instruments. Int J Oral Maxillofac Implants. 1998;13:91-96.
- Yen Nee W, Raja Awang RA, Hassan A. Effects on the titanium implant surface by different hygiene instrumentations: a narrative review. Cureus. 2022;14:e30884.
- Fakhravar B, Khocht A, Jeffries SR, Suzuki JB. Probing and scaling instrumentation on implant abutment surfaces: an in vitro study. Implant Dent. 2012;21:311-316.
- Mann M, Parmar D, Walmsley AD, Lea SC. Effect of plastic-covered ultrasonic scalers on titanium implant surfaces. Clin Oral Implants Res. 2012;23(1):76-82.
- Okubo T, Ikeda T, Saruta J, Tsukimura N, Hirota M, Ogawa T. Compromised epithelial cell attachment after polishing titanium surface and its restoration by uv treatment. Materials (Basel). 2020;13:3946.
- Guo T, Gulati K, Arora H, Han P, Fournier B, Ivanovski S. Race to invade: Understanding soft tissue integration at the transmucosal region of titanium dental implants. Dent Mater. 2021;37:816-831.
- Louropoulou A, Slot DE, Van der Weijden F. Influence of mechanical instruments on the biocompatibility of titanium dental implants surfaces: a systematic review. Clin Oral Implants Res. 2015;26:841-50.
This information originally appeared in Kim SJ, Wadhwani C, Ferracane J, O’Brien R, Katancik JA. The impact of dental probe wear on implant health. Decisions in Dentistry. 2024;10(4):32-35.

