
Treatment of Peri-Implant Mucositis
A comprehensive nonsurgical approach is the best defense against this aggressive threat to gingival health.
Implant gingivitis, better known as peri-implant mucositis, is defined as a reversible inflammatory process in the soft tissue surrounding an osseointegrated dental implant without the loss of marginal bone beyond normal resorption.1 Like gingivitis around natural teeth, the etiology of this disease is related to biofilm accumulation in the soft tissues surrounding titanium dental implants, combined with a susceptible host response.2
Past in vivo human and animal studies have confirmed that peri-implant mucositis is comparable to gingivitis around teeth, both from a histological and clinical perspective.3 Pontoriero et al4 studied 20 partially edentulous patients who received implant therapy. After 6 months of supervised oral hygiene, the patients were asked to stop all oral hygiene practices for 3 weeks—after which plaque control was resumed. When compared with natural teeth, no differences in plaque index, gingival index, or pocket depths were noted.4 Another study looked at the differences in the inflammatory response between natural teeth and implants after 3 weeks of suspended oral hygiene, followed by a return to optimal plaque control.5 Biopsies of the connective tissue were taken, which revealed similar increases in both B lymphocyte and T lymphocyte volume.5
The literature shows that the magnitude and severity of tissue inflammation around an implant experiencing peri-implant mucositis is similar to the dento-gingival complex. More recent studies, however, suggest that the inflammatory process around dental implants may be more severe and more difficult to reverse with treatment. Salvi et al6 looked at experimentally induced mucositis around implants that was treated through nonsurgical mechanical debridement. Unlike previous studies, this revealed that the bacterial challenge presented by the cessation of oral hygiene elicited a greater inflammatory response in the peri-implant mucosa compared to gingiva around the teeth. And, though signs of inflammation decreased after treatment, reversibility did not achieve pre-experimental levels, as noted by inflammatory biomarkers (IL-1B and MMP-8) in the crevicular fluid.6
Schierano et al7 demonstrated the reversal of tissue inflammation to pre-experimental levels was possible after oral hygiene care was suspended and subsequently reinstituted—though it took three times as long as natural teeth (69 days vs 21 days). In summary, the latest studies to examine peri-implant mucositis show that the degree and severity of inflammation are greater and disease reversal more difficult when found near implants compared to tissues proximate to natural teeth.
PREVALENCE
Peri-implant mucositis is common among patients not enrolled in a regular periodontal maintenance program. Among implant patients observed for a 9-year to 14-year period, 48% experienced peri-implant mucositis.8 A similar study revealed that the incidence of peri-implant mucositis can be significantly reduced when patients are involved in a supportive periodontal therapy program.9 As implant gingivitis can be widely prevalent, progress quickly, and become more difficult to resolve than gingivitis around natural teeth, an aggressive nonsurgical approach must be instituted upon diagnosis. In addition, affected patients should be placed on a strict maintenance protocol.
ANTI-INFECTIVE THERAPY
With regard to peri-implant mucositis, anti-infective therapy in the form of mechanical debridement, with or without local/systemic antibiotics, has been shown to be effective during in vivo studies of both humans and animals. Trejo et al10 showed that mechanical debridement with or without the adjunctive use of chlorhexidine resolved mucosal inflammation in monkeys affected by peri-implant mucositis, both clinically and histologically. A study of 25 patients—in which successful treatment was defined as implant survival with no mean pocket depths greater than 5 mm and no further bone loss—reported that 84% of participants treated with nonsurgical mechanical debridement and an adjunctive tetracycline fiber had successful treatment outcomes 12 months post-therapy.11 A study by Salvi et al12 reported that 76% of patients with implants affected by mucositis lesions had successful resolution at 12 months with post-nonsurgical mechanical debridement in conjunction with 1 mg minocycline spheres. While these studies demonstrated a significant reduction in bleeding on probing and pocket depths, complete resolution of the lesion was not achieved, with 41%11 and 44%12 of the sites still showing bleeding on probing.
The most important determinants of successful outcomes when using nonsurgical mechanical debridement to treat peri-implant lesions are the initial depth of the lesions, the amount of bone loss associated with the lesions, and the ability to fully debride implant surfaces. Nonsurgical therapy appears most effective when the lesion is confined solely to the soft tissue at sites with no bone loss. A consensus has not been achieved with regard to nonsurgical therapy when bone loss has occurred. Some studies suggest that nonsurgical interventions are effective when bone loss is present, but only when it is less than 25% of the implant length.13
TREATMENT PROTOCOLS
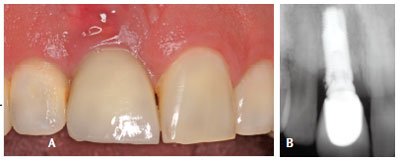
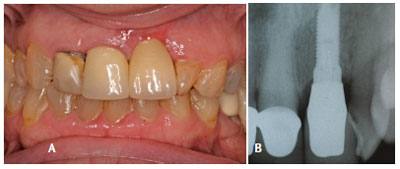
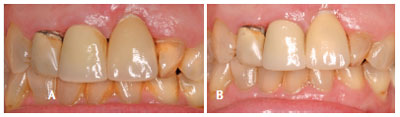
Determining the etiology of the lesion is the first step in developing a treatment plan for peri-implant mucositis. When plaque biofilm is the definitive cause, a diagnosis can be made according to the classification of the peri-implantitis lesions published by Froum and Rosen.14 If a lesion is confined to the soft tissue without bone loss (Figure 1A and Figure 1B), and complete implant surface decontamination is possible, needle-free sulcular anesthesia is administered with a combination of benzocaine/butamben/tetracaine. Mechanical debridement with appropriate titanium curets is then performed. Doses of 1 mg minocycline spheres are placed on the mesial and distal of the implant lesion. The patient is also prescribed systemic antibiotics for 10 days, consisting of 500 mg of metronidazole and 500 mg of amoxicillin twice daily. Follow up is performed at 2-week and 4-week intervals. This protocol has proven successful in resolving inflammation, although there are still exceptions. As seen in Figure 2A and Figure 2B, gingival recession is present at both the 4-week and 12-month intervals. Patients must be advised about this possible sequelae prior to receiving treatment. If, however, the inflammation has not resolved after 1-month of nonsurgical therapy (Figure 3A and Figure 3B), surgical intervention may be deemed necessary (flap surgery, with or without regenerative therapy). A referral to a periodontist is recommended.
CONCLUSION
Peri-implant mucositis is common among implant patients and poses a significant threat to the success of the therapy and the health of patients. While research shows that peri-implant mucositis is similar to gingivitis among those with natural teeth, some studies indicate that the effects of peri-implant mucositis are even more devastating. By determining the etiology of the lesion and developing an effective nonsurgical treatment plan, oral health professionals can reverse the disease process and prevent bone loss.
REFERENCES
- Berglundh T, Lindhe J, Marinello C, Ericsson I, Liljenberg B. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin Oral Implants Res.1992;3:1–8.
- Ericsson I, Berglundh T, Marinello C, Liljenberg B, Lindhe J. Long-standing plaque and gingivitis at implants and teeth in dogs. Clin Oral Implants Res.1992;3:99–103.
- Salcetti JM, Moriarty JD, Cooper LF, et al. The clinical, microbial, and host response characteristics of the failing implant. Int J Oral Maxillofac Implants.1997;12:32–42.
- Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res.1994;5:254–259.
- Zitzmann NU, Berglundh, Marinello CP, Lindhe J. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28:517–523.
- Salvi GE, Agiletta M, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182–190.
- Schierano G, Pejrone G, Brusco P, et al. TNF-a, TGF-b2, and IL-1B levels in gingival crevicular fluids before and after de novo plaque accumulation. J Clin Periodontol. 2008;35: 532–538.
- Roos-Jansåker AM, Renvert H, Lindahl C, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part 1: implant loss and associations to various factors. J Clin Periodontol. 2006;33:283–289.
- Rodrigo D, Martin C, Sanz M. Biologic complications and peri-implant clinical and radiographic changes at immediately placed dental implants. A prospective 5-year cohort study. Clin Oral Implants Res. 2012;23:1224–1231.
- Trejo PM, Bonaventura G, Weng D, Caffesse RG, Bragger U, Lang NP. Effect of mechanical and antiseptic therapy on peri-implant mucositis: an experimental study in monkeys. Clin Oral Implants Res. 2006;17:294–304.
- Mombelli A, Feloutzis A, Brägger U, Lang NP. Treatment of peri-implantitis by local delivery of tetracycline. Clinical, microbiological and radiological results. Clin Oral Implants Res. 2001;12:287–294.
- Salvi GE, Persson GR, Heitz-Mayfield LJ, Frei M, Lang NP. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: clinical and radiographic outcomes. Clin Oral Implants Res. 2007;18:281–285.
- Schwarz F, Bieling K, Bonsmann M, Latz T, Becker J. Nonsurgical treatment of moderate and advanced periimplantitis lesions: a controlled clinical study. Clin Oral Investig. 2006;10:279–288.
- Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32:533–540.
From Dimensions of Dental Hygiene. February 2014;12(2):26,28–29.

