
Tips for Effective Surface Disinfection
Adhering to regulations and choosing the appropriate product are key to reducing the risk of cross-contamination.
Most health care settings rely on chemical disinfection to support their infection prevention efforts. Heat sterilization methods, such as autoclaving and dry heat sterilization, destroy all forms of microbial life. These processes should be used for all heat-stable instruments and devices after patient use. But some equipment and surfaces that can become contaminated during patient care cannot be removed for sterilization or would not survive the heat sterilization processes. Heat-sensitive medical and dental equipment are sometimes immersed in high-level disinfectants or chemical sterilants for reprocessing.
Clinical surfaces that are touched with gloved hands or subjected to contaminated spray or spatter during patient care must be cleaned and disinfected between patients. These surfaces may include: patient chairs, operator and assistant chairs, dental units, small portable equipment, and radiography equipment. As it is not possible to heat sterilize these items once they become soiled, they are most often decontaminated using hospital disinfectants. With no reliable method to confirm the disinfection processes have been successful, it is imperative that the oral health professionals performing these procedures know what products are available; alternatives for special surfaces and equipment; and the proper application and use of the disinfectants and cleaners needed to maintain an aseptic dental care environment.
SURFACES IN DENTAL CARE ENVIRONMENTS
Clinical settings are divided into two types: housekeeping surfaces and clinical contact surfaces. Housekeeping surfaces include floors, walls, and sinks, which carry little risk of disease transmission.1 The majority of these surfaces require only routine cleaning with detergent or water. If the degree of contamination or type of soil present on these surfaces is unknown, a low-level United States Environmental Protection Agency (EPA)-registered hospital-level disinfectant/detergent intended for general housekeeping purposes may be used.
Clinical contact surfaces come into direct contact with oral health professionals’ contaminated gloves or are subject to contamination from the spray, spatter, and aerosol generated during dental procedures.1 When not properly decontaminated between patients, these surfaces can carry the risk of cross-contamination between patients and between patients and dental personnel. Examples of clinical contact surfaces include: light handles and switches, computers, drawer pulls, countertops, X-ray equipment, nitrous oxide equipment, and reusable containers of dental materials.
Some surfaces, such as headrests with deep seams, digital radiographic sensors, endodontic microscopes, and computer keyboards, are difficult to clean and disinfect with chemical germicides due to their shape, size, or sensitivity to moisture or chemicals. These items should be carefully covered with fluid-resistant barriers during patient use. The barriers should be changed between patients and handled carefully to avoid the risk of cross-contamination. If the barrier tears or the surface is inadvertently contaminated, the surface should be cleaned and disinfected before replacing barriers.1
CLASSIFICATIONS OF DISINFECTANTS
Two separate federal agencies regulate manufacturer claims regarding the efficacy and safety of disinfectants. The US Food and Drug Administration (FDA) registers products that are sterilants/high-level disinfectants, and the EPA regulates products that are hospital-level surface disinfectants.1
Sterilants/high-level disinfectants are used only for the reprocessing of critical and semicritical instruments and devices.1 Examples of these products include glutaraldehyde, ortho-phthalaldehyde, and hydrogen peroxide formulations.2 Items are first cleaned in an ultrasonic cleaner, washer/disinfector, or by hand, and then immersed in the high-level disinfectant or sterilant for the amount of time indicated in the instructions for use. These products should be used in covered containers in well-ventilated areas. The active chemicals in these products may cause skin sensitivity, and the safety data sheet provided by the manufacturer or distributor should be reviewed prior to use. High-level disinfectants should never be used on environmental surfaces.1 In addition, some of the automated cleaning processes now available have been cleared by the FDA as capable of achieving high-level disinfection. These products are classified as instrument washer/disinfectors. Instrument washers that simply clean instruments and do not disinfect them are also available. Commercial household dishwashers are not FDA-approved for this purpose.
CAMR / A. BARRY DOWSETT / SCIENCE SOURCE
Clinical surface disinfectants are registered by the EPA as hospital-level disinfectants. Hospital-level disinfectants encompass both low- and intermediate-level disinfectants, which are defined by the US?Centers for Disease Control and Prevention (CDC) in its publication Guidelines for Infection Control in Dental Health-Care Settings—2003.1
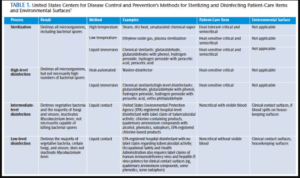
The CDC identifies three levels of disinfectants based on their ability to kill resistant organisms (Table 1). High-level sterilants/disinfectants may be used for heat-sensitive critical items and are capable of killing highly resistant spores under the correct circumstances, such as prolonged immersion, usually within 10 hours to 12 hours.2 Intermediate-level disinfectants are tuberculocidal and kill a broad spectrum of microorganisms, including lipid and nonlipid viruses, vegetative bacteria, and most fungi. Low-level disinfectants kill viruses such as hepatitis B and human immunodeficiency virus, some vegetative bacteria, and fungi, but are not effective against Mycobacterium tuberculosis (Figure 1). The CDC recommends the use of intermediate-level tuberculocidal disinfectants that are EPA-registered for clinical contact surfaces visibly contaminated with blood or other potentially infectious materials. For clinical contact surfaces and equipment not visibly contaminated with blood or other potentially infectious materials, either a low- or intermediate-level disinfectant may be used.1
HOW DISINFECTANTS WORK
Decontamination of clinical surfaces is accomplished through the combination of cleaning and chemical action. Cleaning—the first critical step of the decontamination process—removes debris that would interfere with the action of the chemical germicide and also reduces the number of microorganisms. If cleaning is inadequate, it is possible for enough soil or debris to remain on a surface to interfere with the disinfection process. Disinfectants complete the process by inactivating microorganisms through various mechanisms, depending on disinfectant type.3
Bacteria are destroyed when chemical germicides target their critical structure. Depending on the chemical, the action may affect the cell wall, outer membrane, cytoplasmic membrane, nucleic acids or enzymes, and protein.3 Fungi are also inactivated when germicides target their cell walls, plasma membrane, ribosomes, ribonucleic acid, DNA, or structural and functional proteins. The exact mechanism of how fungicides work to destroy the fungi is not well understood.3
Viruses are smaller than other organisms and are composed of a simpler structure, resulting in fewer targets for the chemical germicides. Germicides target the virus envelope (if present), capsid, and viral genome. The capsid is the structure-giving element of a virus and also protects the viral nucleic acid, which is its infectious component. For a germicide to be virucidal, it must be capable of destroying the viral envelope or capsid, in addition to inactivating viral nucleic acid.3
LIMITATIONS OF DISINFECTANTS
click to view
The effectiveness of surface disinfectants may be hindered by numerous factors. In addition, the manner in which the chemical is applied, contact with certain materials found in towels, improper mixing, incorrect or inadequate application on the surface, insufficient time in contact with the clinical surface, presence of debris or biological material, and other factors may prevent the chemical disinfectant from destroying microorganisms on clinical contact surfaces.1
Although the thoroughness of disinfection procedures has not been well-studied in dental offices, numerous studies in hospitals have demonstrated that adequate environmental cleaning is often lacking.4,5 Personnel often fail to perform cleaning and disinfection following discharge of a patient or perform these procedures improperly, resulting in residual surface contamination.5
In recent years, the use of premoistened disinfectant wipes has gained popularity due to their relative ease of application.6 It is important to use proper technique when cleaning and disinfecting surfaces with wipes. Studies indicate that it is possible to transfer microorganisms from one surface to another in the clinical environment when more than one surface is wiped with a single towel.7 Also, some wipes contain relatively high concentrations of alcohol and may become dry if the container is left open. Some surface disinfectants indicate a contact time of 10 minutes on the product label. The typical dry time for a water-based disinfectant is 1.5 minutes to 2 minutes.4 While there is no evidence of enforcement action, the EPA is clear that failure to follow manufacturer instructions for contact time could result in liability by the user and enforcement action under the Federal Insecticide, Fungicide and Rodenticide Act, under which disinfectants are regulated.8
ALTERNATIVE MEANS OF SURFACE DISINFECTION
An increasing number of materials are being constructed with antimicrobial properties embedded in the product. These include heavy metals, such as silver and copper, which have inherent anti-infective properties.9 Products impregnated or coated with chemical germicides, including triclosan and quaternary ammonium compounds, are also available. These ingredients have been widely used in household products, such as soaps, deodorants, toothpastes, and cutting boards.
A number of alternative technologies for disinfection of clinical contact surfaces are being explored. These are currently used in hospital settings and appear to have limited applicability to outpatient clinical settings, such as dental offices. One such technology is the use of ultraviolet (UV) irradiation. At certain wavelengths, UV light will break the molecular bonds in DNA, destroying the organism. An automated mobile UV unit can be used in a patient room after discharge to eliminate vegetative bacteria, including methicillin-resistant Staphylococcus aureus (MRSA, Figure 2).10 Another technology involves the use of hydrogen peroxide. These systems produce hydrogen peroxide vapor or aerosolized dry mist hydrogen peroxide. One study showed a hydrogen peroxide vapor machine used in a patient room completely inactivated Bacillus stearothermophilus (the spore used in biological indicators to validate sterilization processes) and MRSA from surfaces.11 The use of these technologies is impractical in modern dental practice because they require prolonged time to deploy, must be used in a room where no one is present, and necessitate precleaning of surfaces to remove debris and soil.10 However, it is worthwhile to monitor the evolution of products and devices used in hospitals, as these technologies may find their way into outpatient settings in the future.
At this time, oral health professionals need to rely on FDA- or EPA-approved sterilants/disinfectants and adhere to all manufacturer instructions when in use. Surface disinfection is just one part of an effective infection control protocol, and all recommendations for this aspect, as well as all other facets of infection control provided by the CDC’s Guidelines for Infection Control in Dental Health-Care Settings should be followed in order to ensure patient and clinician safety.
REFERENCES
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52:1–61.
- Rutala WA, Weber DJ. Disinfection and sterilization: an overview. Am J Infect Control. 2013;41:S2–S5.
- Russell AD. Principles of antimicrobial activity and resistance. In Block SS, ed. Sterilization, Disinfection and Preservation. 5th ed. Philadelphia; Lea & Febiger; 2001:31–55.
- Rutala WA, Weber DJ. Disinfectants used for environmental disinfection and new room decontamination technology. Am J Infec Cont. 2013;41(Suppl 5):S36–S41.
- Carling PC, Perry MF, Von Beheren SM. Identifying opportunities to enhance environmental cleaning in 23 acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:687–699.
- Wiemken TL, Curran DR, Pacholski EB, et al. The value of ready-to-use disinfectant wipes: compliance, employee time, and costs. Am J Infect Control. 2014;42:329–330.
- Williams GJ, Denyer SP, Hosein IK, Hill DW, Maillard JY. Limitations of the efficacy of surface disinfection in the healthcare setting. Infect Control Hosp Epidemiol. 2009:6;570–573.
- Rutala WA, Weber DJ. Healthcare Infection Control Practices Advisory Committee. Guideline for disinfection and sterilization in healthcare facilities, 2008. Available at: cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov _2008.pdf. Accessed May 21, 2014.
- Weber DJ, Rutala WA. Self-disinfecting surfaces: review of current methodologies and future prospects. Am J Infect Control. 2013;41(Suppl 5):S31–S35.
- Fornwalt L, Riddell B. Implementation of innovative pulsed xenon ultraviolet (PX-UV) environmental cleaning in an acute care hospital. Risk Manag Healthc Policy. 2014;7:25–28.
- French GL, Otter Ja, Shannon KP, Adams NM, Watling D, Parks MJ. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapor decontamination. J Hosp Infect. 2004;57:31–37.
From Dimensions of Dental Hygiene. June 2014;12(6):26–30.

