 MICROSCAPE / SCIENCE SOURCE
MICROSCAPE / SCIENCE SOURCE
Screening for Oral Cancer
Early detection is key to reducing the mortality and morbidity associated with this serious disease.
This course was published in the December 2015 issue and expires December 21, 2018 2021. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the prevalence of oral cancer.
- Identify the most frequent locations of oral cancer lesions.
- List changes in the oral mucosa caused by oral cancer.
- Explain the adjuncts designed to increase the likelihood of early oral cancer detection.
CLINICA CLAROS / SCIENCE SOURCE
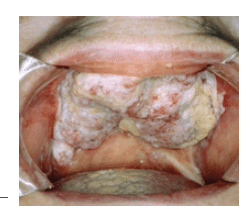
Currently, oral cancer is the eighth most common cancer worldwide, with 300,000 new cases reported every year.2 In the United States, approximately 41,000 new cases of oral and oropharyngeal cancers were reported in 2013, causing an estimated 7,500 deaths in that year.3 Men are at greater risk of the disease than women. The median age of those diagnosed is 62, although the number of oral and oropharyngeal cancers diagnosed in young adults is growing due to increasing rates of human papillomavirus (HPV)-infection.3 HPV encompasses more than 100 types of double-stranded DNA viruses of the papovavirus subgroup. Infection with HPV causes papillary lesions on various areas of the body, including mucosal tissues of the head and neck (Figure 1).4,5 Infection with HPV?strains 16 or 18 increases the risk of oral and oropharyngeal cancers, which are most often found in young nonsmoking, nondrinking adults. Oral HPV?infection most commonly occurs through oral-to-genital contact. It remains unclear if deep kissing can cause HPV infection. Additional risk factors include young age at first intercourse, history of sexually transmitted infections, low condom usage, and high number of sexual partners.4,6–8
Tobacco and alcohol use are the most recognized risk factors for cancers of the oral cavity. Risk levels increase 30% when tobacco and alcohol are used together.9 In fact, smokers who are heavy drinkers are 38% more likely to develop oral and oropharyngeal cancers than those who do not engage in these behaviors.3,10
The overall 5-year survival rates for oral cancer have remained low at approximately 50%, likely because most cases are not caught until they are in advanced stages.11 Despite significant progress in cancer treatments, early detection of oral cancer and its curable precursors remains the recommended strategy for ensuring patient survival and improving quality of life. According to Healthy People 2020, detecting oral and pharyngeal cancers at the earliest stages is a critical oral health objective.12 HPV-related cancers are more likely to be successfully treated than other forms of oral cancer.
LESION LOCATION
Oral cavity and oropharyngeal cancers are most often detected on the lateral border of the tongue, tonsils, oropharynx, and floor of the mouth. Other areas that should be examined include the lips and minor salivary glands.3 HPV-related oral cancers are most commonly found at the base of the tongue and on the tonsils.13 Oral cavity cancers frequently occur at sites of dental trauma, especially among nonsmokers without other risk factors. Dental trauma/irritation, however, is not recognized as an oral cancer risk factor.14
CHANGES IN ORAL MUCOSA
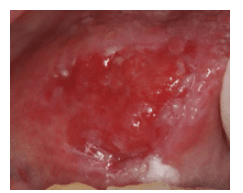
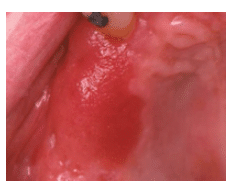
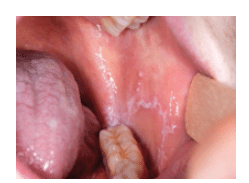
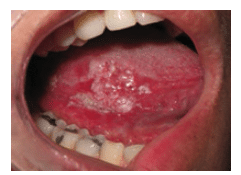
Virtually all oral squamous cell carcinomas are preceded by visible changes in the oral mucosa, usually appearing as leukoplakia (white) or erythroplakia (red) patches (Figure 2 and Figure 3). Other conditions, such as erosive lichen planus (Figure 4) and submucous fibrosis, are associated with an increased risk of oral squamous cell carcinoma (Figure 5). Capillary transformation occurs prior to tumor growth. The pattern of tumor angiogenesis (formation of new blood vessels from preexisting vessels) is different from that seen in neovascularization during the repair and regeneration processes.15
Dental hygienists must note any change in a patient’s oral cavity. Documentation should include the history of the lesion, location, clinical description, and morphology, such as fluid-filled, depressed, or ulcerated. Re-evaluation of the lesion should be performed at 2 weeks. If the lesion remains, a scalpel biopsy of the lesion should be performed. If a lesion appears highly suspicious at the time of first detection, however, immediate referral for biopsy is indicated.
EARLY DETECTION
Intraoral and extraoral visual and tactile examination are the basis of oral cancer screening.3,10,15–32 Traditional oral cancer screening includes recording an updated medical and dental history to identify risk factors such as tobacco use, alcohol consumption, HPV infection, and genetic influence.16 The similarity between the clinical appearance of benign and premalignant oral lesions makes it impossible to rely on traditional oral cancer screening alone; scalpel biopsy and histopathological assessment are needed to make a definitive diagnosis. As such, the demand for additional tools to aid in oral cancer screening is high. Safe and cost-effective adjunct technologies may improve diagnosis and early treatment, which could decrease mortality rates and improve quality of life.10,16 However, no screening test is 100% accurate, meaning that some cancers may go undetected, while innocuous tissues may be suspect. The gold standard for oral cancer diagnosis remains tissue biopsy with a pathological assessment.15,22,24
There are several types of adjuncts available to support oral cancer risk assessment and lesion detection and assessment, including: salivary diagnostics; visual adjuncts such as tissue reflectance devices and autofluorescence devices; and tests based on transepithelial cytology.33
SALIVARY DIAGNOSTICS
Salivary diagnostics is used to detect a patient’s specific mRNA transcriptome markers in saliva.20 One test uses electrochemical detection of salivary proteins and nucleic acids to note biomarkers that change their expression characteristics in oral squamous cell carcinoma. The test can be conducted chairside and results are available within 15 minutes. Patients who receive positive results are then referred for biopsy and pathological assessment.
A new risk assessment system not yet available in the US also utilizes salivary diagnostics. The products, which include a laboratory test and a point-of-care test, look for the specific protein markers that demonstrate an increased risk of oral cancer. Patients with elevated risk levels can be counseled to make lifestyle changes to reduce their oral cancer risk. The tests are also designed to support the early detection of oral cancer.34 Salivary diagnostics may be an ideal screening technique for the detection of premalignant and malignant lesions in the oral cavity.35,36
VISUALIZATION ADJUNCTS
Visualization adjuncts are designed to penetrate epithelial tissue, enabling clinicians to examine changes in the characteristics of oral tissues.29 This is because abnormal mucosa tissues may absorb and reflect light differently than healthy tissue. Some systems incorporate the use of acetic acid mouthrinses to first desiccate the mucosa, in efforts to make tissue abnormalities more visible. These adjuncts can be used in conjunction with visual examination to enhance or clarify findings.
Screening devices based on tissue reflectance use an additional light source to find differences in the oral mucosa that may be challenging to detect in traditional treatment room conditions.33 One system incorporates vital tissue staining in addition to the light source. The liquid tolonium chloride is applied to the tissue via mouthrinse and then decolorized with the administration of acetic acid. The tissues are then examined with a handheld light source. The dye appears clear when applied to healthy cells, but is thought to adhere to abnormal cells, alerting the clinician to their presence.28,29 Vital tissue staining has been used for years in the detection of malignancies with acetic acid in the cervical mucosa (PAP smear).18
Autofluorescence has been used to aid in the detection of early cancerous or precancerous lesions since the early 1990s.24 Autofluorescence is used to identify lesions that exhibit changes in tissue density. A light source and handpiece are used to examine the tissue, with healthy tissue appearing green and abnormal tissue appearing dark in comparison.33
Clinicians should be aware of any intraoral variants of normal before using autofluorescence to prevent false positives. Once a lesion is detected by fluorescence and its cause cannot be determined, the oral health professional refers the patient to a specialist to biopsy the area. Fluorescence can be used to help delineate the location of the lesion, including borders, which may be helpful when the biopsy is conducted.22,27 Another system combines tissue reflectance and autofluorescence to note areas of concern.
TRANSEPITHELIAL CYTOLOGY
The brush test is based on transepithelial cytology.28,29 It uses a specially designed brush to remove a sample of transepithelial surface cells that are sent to a lab where they are screened by a pathologist. Results are labeled negative/benign, positive, or atypical. Once the results are returned to the dentist, he or she determines the best course of action. A positive result typically results in biopsy.
EVIDENCE-BASED RECOMMENDATIONS
The literature supports the use of visual and tactile examination to detect oral cancer in its earliest stages, especially among patients at high risk, including tobacco and heavy alcohol users.33 Unfortunately, many oral health professionals fail to perform oral cancer examinations on a biannual basis.
The evidence on the use of adjuncts in the detection of oral cancer is mixed. In a 2010 review, the American Dental Association (ADA) Council on Scientific Affairs Expert Panel on Screening for Oral Squamous Cell Carcinoma reported that the evidence is insufficient to recommend the use of tissue reflectance, autofluorescence, or transepithelial cytology as effective adjuncts to the traditional visual and tactile examination. The review did find that when testing oral lesions with a high potential for malignancy, transepithelial cytology was effective at finding dsyplastic cells, but questions regarding whether such lesions ought to be biopsied as a matter of routine remain.33 The use of salivary diagnostics was not included in the ADA review, but a literature review by Yakob et al36 noted that additional high-quality studies are needed that incorporate proteomic, transcriptomic, methylomics, and others to enable the best use of saliva in detecting oral squamous cell carcinoma.
FUTURE CONSIDERATIONS
More research is needed to further investigate the usefulness of adjunct technologies in the early detection of oral cancer. They may offer the greatest value when used on patients with the highest number of risk factors. At this time, oral health professionals must remain vigilant regarding the performance of frequent visual and tactile examinations to detect oral cancer in its earliest stages. The early diagnosis of oral cancer is critical to the ability to use less invasive treatments, improving quality of life, and potentially reducing mortality rates. Delays in the detection, diagnosis, and treatment of oral cancer are associated with survival rates of less than 5 years, further suggesting that early detection and treatment will improve prognoses.10
REFERENCES
- Ford PJ, Farah CS. Early detection and diagnosis of oral cancer: Strategies for improvement. Journal of Cancer Policy. 2013;1(1-2):e2–e7.
- Petersen PE. The world oral health report 2003: continuous improvement of oral health in the 21st century—the approach of the WHO global oral health programme. Community Dent Oral Epidemiol. 2003;31(Supp. 1):3–24.
- American Cancer Society. Oral Cavity and Oral Pharyngeal Cancer. Available at: cancer.org/cancer/ oralcavityandoropharyngealcancer/index. Accessed November 9, 2015.
- Terai M, Takagi M. Human papillomavirus in the oral cavity. Oral Med Pathol. 2001;6:1–12.
- Lukes SM, Meneses MB. Dimensions of Dental Hygiene. 2010;8(6):72–77.
- Sharuga CR, Price TP, Dotson D. Educate your patients about HPV. Dimensions of Dental Hygiene. 2012;10(1):52–55.
- D’Souza G, Kreimer AR, Viscidi R, et al. Casecontrol study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356: 1944–1956.
- Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118(Suppl 1):S12–S17.
- Petersen PE. Oral cancer prevention and control—the approach of the World Health Organization. Oral Oncol. 2009;45:454–460.
- Brocklehurst P, Kujan O, O’Malley LA, Ogden G, Shepherd S, Glenny AM. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev. 2013;11:CD004150.
- National Institute of Dental and Craniofacial Research. Oral Cancer 5-Year Survival Rates by Race, Gender, and Stage of Diagnosis. Available at: nidcr.nih.gov/DataStatistics/FindDataByTopic/OralCancer/OralCancer5YearSurvivalRates.htm. Accessed November 6, 2015.
- Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at: healthypeople.gove. Accessed November 6, 2015.
- Centers for Disease Control and Prevention. Human papillomavirus (HPV) and Oropharyngeal Cancer Fact Sheet. Available at: cdc.gov/std/ hpv/stdfact-hpvandoralcancer.htm. Accessed November 6, 2015.
- Perry BJ, Zammit AP, Lewandowski AW, et al. Sites of origin of oral cavity cancer in nonsmokers vs smokers: possible evidence of dental trauma carcinogenesis and its importance compared with human papillomavirus. JAMA Otolaryngol Head Neck Surg. 2015;141:5–11.
- Messadi D, Younai F, Liu H, Guo G, Wang C. The clinical effectiveness of reflectance optical spectroscopy for the in vivo diagnosis of oral lesions. Int J Oral Sci. 2014;6:162–167.
- Ayoub H, Newcomb T, McCombs G, Marshall B. The use of fluorescence technology versus visual and tactile examination in the detection of oral lesions: A pilot study. J Den Hyg. 2015;89:63–71.
- Rana M, Zapf A, Kuehle M, Gellrich NC, Eckardt AM. Clinical evaluation of an autofluorescence diagnostic device for oral cancer detection: A prospective randomized diagnostic study. Eur J Cancer Prev. 2012;21:460–466.
- Rashid A, Warnukulasuryia S. The use of light based (optical) detection systems as adjuncts in the detection of oral cancer and oral potential malignant disorders: A systematic review. J Oral Path Med. 2015;34:307–328.19. McGee S,
- Mardirossian V, Elackattu A, et al. Anatomy-based algorithms for detecting oral cancer using reflectance and fluorescence spectroscopy. Ann Otol Rhinol Laryngol. 2009;118:817–826.
- Malathi N, Mythili S, Vasanthi HR. Salivary diagnostics: a brief review. ISRN Dent. 2014;2014:158786.
- Laronde D, Williams T, Hislop C, et al. In?uence of ?uorescence on screening decisions for oral mucosal lesions in community dental practices. J Oral Pathol Med. 2014;43:7–13.
- Messadi D. Diagnostic aids for the detection of precancerous lesions. Int J Oral Science. 2013;5:59–65.
- Francisco A, Correr W, Azevedo L, et al. Fluorescence spectroscopy for the detection of potentially malignant disorders and squamous cell carcinoma of the oral cavity. Photodiagnosis Photodyn Ther. 2014;11:82–90.
- Onizawa K, Saginowa H, Furuya Y, Yoshida H. Fluorescence photography as a diagnostic measure for oral cancer. Cancer Letters. 1996;108:61–66.
- Hsu ER, Gillenwater A, Richards-Kortum RR. Detection of the molecular changes associated with oral cancer using a molecular-specific contrast agent and single wavelength spectroscopy. Appl Spectrosc. 2005;59:1166–1173.
- Jayaprakash V, Sullivan M, Merzianu M, et al. Autofluorescence-guided surveillance for oral cancer. Cancer Prev Res (Phila). 2009;2:966–974.
- Shin D, Dongsuk V, Vigneswaren N, Gillenwater A, Richards-Kortum R. Advances in fluorescent imaging techniques to detect oral cancer and its precursors. Future Oncology. 2010;6:1143–1154.
- Fedele S. Diagnostic aids in the screening of oral cancer. Head Neck Oncol. 2009;1:5
- Schmidt B. Current topics in oral cancer research and oral cancer screening. J Dent Hyg. 2012;86:7–8.
- Lopez-Journet P, De LaMano-Espinosa T. The efficacy of direct tissue fluorescence visualization in screening for oral premalignant lesions in general practice. Int J Dent Hyg. 2011;9:97–100.
- Sweeney L, Dean N, Magnuson S, Carroll W, Clemons L, Rosenthal E. Assessment of tissue autofluorescence and reflectance for oral cavity cancer screening. Otolaryngol Head Neck Surg. 2011;145:956–960.
- Warnakulasuryia R. The use of light-based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. Oral Pathol Med. 2015;44:307–328.
- Rethman MP, Carpenter W, Epstein J, et al. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. J Am Dent Assoc. 2010;141:509–520.
- Franzmann EJ, Reategui EP, Pereira LH, et al. Salivary protein and solCD44 levels as a potential screening tool for early detection of head and neck squamous cell carcinoma. Head Neck. 2012;34:687–695.
- Patil A, Choudhari K, Unnikrishnan VK, et al. Salivary protein markers: a non-invasive profile based method for the early diagnosis of premailgnancy and malignancy. J Biomed Opt. 2013;18:101317.
- Yakob M, Fuentes L, Wang MB, Abemayor E, Wong DT. Salivary biomarkers for detection of oral squamous cell carcinoma—current state and recent advances. Curr Oral Health Rep. 2014;1:133–141.
From Dimensions of Dental Hygiene. December 2015;13(12):56–59.



