Heliobacter Pylori and the Oral Cavity
Is this gastric infection-causing bacterium related to oral health?
This course was published in the May 2008 issue and expires May 2011. This course was developed in part with an unrestricted educational grant from Colgate Oral Pharmaceuticals. This 2 credit hour self-study activity is electronically mediated.
Educational Objectives
After reading this course, the participant should be able to:
1. Identify the potential relationship between Helicobacter pylori and oral diseases.
2. Understand the mechanism of Helicobacter pylori transmission.
3. Describe the role of dental plaque in Helicobacter pylori infections.
4. Discuss the influence of oral Helicobacter pylori and stomach infections.
5. List the common risk indicators shared by Helicobacter pylori and herpes simplex virus type 1.
Although controversial, a body of evidence has evolved since the mid-1990s suggesting that Helicobacter pylori (HP), a microorganism implicated in many gastric diseases, may be related to a number of oral diseases and conditions. This research is important to practicing dental professionals as it has the potential to influence the treatment of patients with a history of gastric conditions who do not respond to eradication therapy.
ABOUT HELICOBACTER PYLORI
First isolated from human gastric biopsy in 1982,1 HP is a spiral-shaped gram-negative bacterium and one of the most common bacterial infections in humans (Figure 1).2,3 The worldwide occurrence of HP gastric infection reaches as high as 80% in developing countries and 40% in developed countries.4 The prevalence of HP in the United States is estimated to be 25% in the 6- to 19-year-old population and 33% in the adult population.2
In investigating serum samples of a large population of couples attending a New York-based infertility clinic, Perez-Perez and colleagues5 reported that HP infection strongly correlates with place of birth. Of subjects born outside of the United States, 69.1% were HP-seropositive compared with 8.7% of subjects born in the United States. The infection is typically acquired early in life. The age of acquisition is geographically variable2,6 and studies show a familial clustering of HP infection.7-10
HP is closely linked to chronic gastritis and peptic ulcer among other more serious conditions.3 The organism is designated as a type 1 carcinogen, predisposing those infected to gastric cancer and mucosa-associated lymphoid tissue lymphoma.4,11 Because HP infection is usually asymptomatic, clinical findings alone are not sufficient in determining when the infection occurs.2 Following infection of HP, immune responses usually develop. Antibody levels often decline slowly after treatment of the organism.2
HP’s mechanism of transmission is still unclear, although we do know that humans and primates are the predominant conduit2 in which both oral-oral, fecal-oral, and gastro-oral (vomit) transmission occurs.2,4 Accordingly, the main routes of transmission appear to be through infected saliva or contaminated food and eating utensils.4 Various studies also report a high degree of association between the handling of animals, particularly the domestic cat,12 and HP infection, which indicates that animals (including sheep and the housefly)2 may play a role in HP transmission.3 Given the probability of saliva transmission, the idea that the oral cavity may play a pivotal role in the transmission of HP seems highly plausible. Nonetheless, the precise role of the oral cavity in transmitting this microorganism is the subject of controversy.4
Photo © MedicalRF.com/Visuals Unlimited
Numerous hypotheses exist regarding the pathological significance of HP within the oral environment. For instance, HP may be transiently present in the oral cavity leading to an unbalance of the residual flora.4 Another related theory is that HP is part of a normal oral microenvironment that is not a pathogenic reservoir of HP in the stomach, but rather a component of a normal immunological balance that may even provide protection against dangerous pathogens.4
It is also possible that the quantities of HP colonizing the oral cavity are too small to cause gastric infection as long as the host immunity remains intact. If the host’s immunological defense becomes impaired, the bacterium’s role as a commensal organism (parasitic organism that causes no harm to the host) may change to pathogenic.4
Despite the current treatment regimens that lead to successful management of HP-positive chronic gastritis, the reinfection rate is relatively high.13 Numerous studies have hypothesized that the oral cavity may be a reservoir for HP infection and that oral secretions may be a significant means of transmission of the organism.3 The bacteria have been detected in saliva and on the tongue, leading to the theory that recolonization of HP from dental plaque may be one mechanism of reinfection.3
Studies addressing the possibility that the oral flora could be a permanent reservoir of viable HP and that the organism, harbored within dental plaque, can be implicated in HP-associated gastric conditions, are inconclusive.3 Gastric-related HP has been implicated in halitosis14,15 and the microorganism has also been linked to increased risk for herpes simplex virus type 1 (HSV-1).2 However, not all reports16 investigating these associations are in agreement.
ORAL CAVITY—A RESERVOIR FOR HP?
The major question regarding the presence of HP in the oral cavity is whether the organism is a transient bacterium, part of the normal biofilm, or whether it plays a pathogenic role. Studies regarding the oral localization of HP are conflicting. Numerous reports indicate the occurrence of HP within the oral cavity ranges from 38% of subjects to 100%, depending on study populations, sample collection, and laboratory procedures.4 Some researchers believe that HP should be categorized within the normal microflora of the oral cavity.17,18 Others suggest that the presence of HP in the oral cavity is temporary and possibly related to occupational exposure to the microorganism.19,20 The temporary existence of HP in dental plaque may also be related to gastroesophageal reflux.21,22
Numerous studies have examined the role of dental plaque in HP infection. Anand and colleagues3 found a higher prevalence of HP in the dental plaque of patients with gastric HP infection than patients who were negative for HP. Similar results regarding the high prevalence of HP in dental plaque have been reported in other studies.23-25
Dental plaque may be a permanent reservoir of HP.26,27 Evidence suggests that HP may be present in supra- and subgingival plaque28 and in sites of periodontitis.29-31 Other research appears to conflict with these findings.32,33 Kignel and colleagues34 reported that supragingival dental plaque and saliva may not be relevant reservoirs of HP. It is also possible that HP is not directly implicated in periodontitis, but instead it may co-aggregate with specific microbial pathogens known to cause periodontal diseases.
Okuda and colleagues35 found that Porphyromonas gingivalis and Fusobacterium nucleatum—both periodontal pathogens—are able to adhere to and then trap HP. This offers a possible explanation of why HP is often detected in patients with periodontitis. Some bacteria associated with normal biofilm may actually inhibit the growth of HP.35 This discovery supports the theory that HP occurs transiently within the oral cavity due to its removal by other bacteria within the biofilm.
Some studies suggest that poor periodontal health may be associated with HP infection. By analyzing data obtained from the Third National Health and Nutrition Examination Survey (NHANES III), Dye and colleagues6 reported that periodontal pockets with a depth greater than 5 mm were more likely to have HP present in the blood after adjusting for socioeconomic factors. In a study conducted by Riggio and colleagues36 in plaque specimens acquired from periodontal pockets that were 5 mm or greater, 33% of the cultured sites were positive for HP and 38% of the 29 patients studied had moderate-to-severe periodontal pocketing that was also positive for HP. This led to the conclusion that subgingival plaque in adults with periodontitis may function as a reservoir for HP. Dye and colleagues6 note: “HP survives in moderate to advanced periodontal pockets because the architecture and the microcosm of these periodontal conditions promote a viable habitat for micro-aerophilic and anaerobic microorganisms.” They further hypothesized that because dental biofilms provide urea, the viability of urease-producing bacteria such as HP can be sustained.
GASTRO-ORAL TRANSMISSION
Saliva samples following vomitus are almost three times as likely to contain HP than saliva samples before the vomiting event.37 The theory is that the forces associated with vomiting are capable of impregnating plaque biofilm with ejected HP.6 Accordingly, subgingival plaque harbored within deeper periodontal pockets may promote the colonization and survival of HP.6
Suoto and colleagues38 found that HP was detected frequently in the oral microbiota of subjects with periodontitis, suggesting that periodontal pocketing and inflammation may favor the colonization by this species. Gebara and colleagues31 recently reported that treatment regimens seeking to eradicate HP were more effective in the stomach than the mouth. This led the researchers to conclude that the oral cavities of patients with gingivitis or chronic periodontitis, who also have gastric HP, may be reservoirs of these bacteria.
These studies are in contrast with the research of Anand and his colleagues3 who found little correlation between oral hygiene and periodontitis status and HP infection (after adjusting for sociodemographic variables). This study is in agreement with an earlier study39 that found no association between periodontitis and HP infection. In determining the prevalence of HP in the subgingival plaque of patients with periodontitis, one study suggested that periodontal pockets do not constitute a natural reservoir for HP.40 Martinez-Gomis and colleagues41 recently concluded that HP does not seem to be permanently present in the oral cavity of a nondyspeptic population.
The theory that gastric-related HP can be translocated to the oral cavity through vomitus and back to the gut from the oral cavity has spawned discussion on the control and eradication of HP infection. Miyabayashi and colleagues42 found the success rate for gastric eradication to be significantly related to the prevalence of HP in the oral cavity. Evidence also exists showing that eradication treatment does not appear to affect the oral cavity because of the resistance of dental plaque’s host-associated biofilm.43 Since systemic eradication of HP from dental plaque has not been successful, local treatment modalities aimed at plaque control may be indicated to prevent recolonization of HP of the stomach.13
HP IN PLAQUE VS THE STOMACH
In investigating whether the oral cavity is a potential reservoir or possible sanctuary for HP, Nguyen and colleagues27 concluded that HP colonization is not restricted to the gastric mucosa. They further reasoned that the ecological niche of the oral cavity may serve as a potential sanctuary for HP, increasing the possibility for re-infection of the gut after the end of therapy due to the recolonization of the stomach from HP present in dental plaque. Umeda and colleagues30 concluded that close attention should be given to periodontitis patients who harbor HP in the oral cavity. In order to successfully eradicate HP, medical-dental collaboration in coordinating care for those with HP infection may be indicated.
In their study of a relatively homogeneous population of 88 patients with symptoms of dyspepsia (discomfort from indigestion that may be a sign of various underlying intestinal disorders such as peptic ulcer, gallbladder disease, or chronic appendicitis), Chitasazi and colleagues44 concluded that an association did not exist between HP in dental plaque and the stomach as potential sources of this microorganism. These findings mirrored those of other researchers.45-48
 Some researchers have insisted on a positive association between HP in dental plaque and the stomach.49,50 The same strain of HP found in the stomach has been identified in subgingival plaque.51 A higher prevalence of HP infection in men, both in dental plaque and the stomach, has been reported.44,52 Patients with upper abdominal complaints may have a higher prevalence of HP colonization within the oral cavity.53
Some researchers have insisted on a positive association between HP in dental plaque and the stomach.49,50 The same strain of HP found in the stomach has been identified in subgingival plaque.51 A higher prevalence of HP infection in men, both in dental plaque and the stomach, has been reported.44,52 Patients with upper abdominal complaints may have a higher prevalence of HP colonization within the oral cavity.53
In evaluating the influence of oral HP on stomach infection and the release of gut hormones affecting food intake such as ghrelin (a hormone produced in the stomach that stimulates appetite) and gastric secretions, Czesnikiewicz-Guzik and colleagues4 found that oral HP alone did not serve as a harbor for gastric HP infection and the presence of HP in the oral cavity does not appear to induce appetite alterations. In the same study, researchers reported that the presence of HP in the oral cavity did not seem to be associated with periodontal health status.
HP AND HERPES SIMPLEX VIRUS TYPE 1
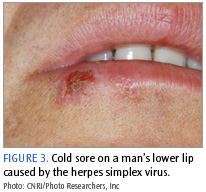
Humans are the only known reservoir of herpes simplex virus type 1 (HSV-1), another very common infection (Figure 2). Direct human-to-human contact is the main form of transmission. The infections have very similar features (Figure 3) including shared risk indicators and modes of transmission.2 In addition, both infections are largely asymptomatic so that the onset, presence, and history can only be established with certainty through the study of blood serum.2 Advanced age, low socioeconomic status or educational level, and crowded living conditions are risk indicators shared by both HP and HSV-1. In the United States, HP and HSV-1 are more prevalent in African American and Latino populations.2 Both organisms have been isolated from oral and gastric mucosa.2 Robinson and colleagues54 demonstrated an association between the seroprevalence of antibodies against the two organisms.
The findings of Baccaglini and colleagues2 indicate that HSV-1 does not appear to favor HP colonization or vice-versa. Further, their research found an association between HSV-1 and HP that ranged from negligible to strong, based on household size and location. Specifically in small nonurban households, there appeared to be little association. This led to the conclusion that the observed association between HP and HSV-1 is more likely due to shared risk indicators and routes of transmission than direct biological mechanisms.
PRACTICAL APPLICATION
The research related to the potential association between oral HP and gastric-related HP is inconclusive. Nonetheless, is there enough evidence to recommend new clinical directives for patients who present with diagnosed or suspected HP-associated gastric conditions? Even though the evidence is inconclusive, entering into collaborative management of these cases with medical providers to ensure both oral and gastric health is only positive for patients. Following are some suggestions for everyday practice based on the current available research.
- In assessing patients’ risk for periodontitis, inquiring whether there is a history of gastric diseases or conditions—either diagnosed or suspected—is appropriate. For a patient with a gastric condition and periodontitis, dental professionals may want to contact the patient’s physician to determine whether collaborative case management would be beneficial.
- HP in dental plaque is seldom eliminated by HP eradication therapy and, consequently, this reservoir may act as a source for future reinfection.3 Therefore, eradication of HP from dental plaque should be an important part of comprehensive management of HP-associated gastric diseases.3 This includes local treatment modalities such as scaling and root planing and patient education on plaque control.
- The possible association between HP and HSV-1 indicates that their shared risk factors or routes of transmission may play a role in the communicability of these two infections.2 Accordingly, for a patient who presents with HSV-1, dental professionals should consider that HP within the oral cavity may also be present.
REFERENCES
- Christie JM, McNulty CA, Shepherd Na, et al. Is saliva serology useful for the diagnosis of Helicobacter pylori? Gut. 1996;39:27-30.
- Baccaglini L, Schoenbach VJ, Poole C, et al. Association between herpes simplex virus type 1 and Helicobacter pylori in US adolescents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:63-69.
- Anand PS, Nandakumar K, Shenoy KT. Are dental plaque, poor oral hygiene, and periodontal disease associated with Helicobacter pylori infection? J Periodontol. 2006;77:692-698.
- Czesnikiewicz-Guzik M, Bielanski W, Guzik TJ, Loster B, Konturek SJ. Helicobacter pylori in the oral cavity and its implications for gastric infection, periodontal health, immunology and dyspepsia. J Physiol Pharmacol. 2005;56(Supp 6):77-89.
- Perez-Perez GI, Witkin SS, Decker MD, Blaser MJ. Seroprevalence of Helicobacter pylori infection in couples. J Clin Microbiol. 1991;29:642-644.
- Dye BA, Kruszon-Moran D, McQuillan G. The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in the United States. Am J Public Health. 2002;92:1809-1815.
- Drumm B, Perez-Perez GI, Blaser MJ Sherman PM. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990;322:359-363.
- Malaty HM, Graham DY, Klein PD, Evans DG, Adam E, Evans DJ. Transmission of Helicobacter pylori infection. Studies in families of healthy individuals. Scand J Gastroenterol. 1991;26:927-932.
- Malaty HM, Engstrand L, Pederson NL, Graham DY. Helicobacter pylori infection: genetic and environmental influences. A study of twins. Ann Intern Med. 1994;120:982-986.
- Oderada GD, Vaira J, Holton C, et al. Helicobacter pylori in children with peptic ulcer and their families. Dig Dis Sci. 1991;36:572-576.
- Albanidou-Farmaki E, Giannoulis L, Markopoulos A, et al. Outcome following treatment for Helicobacter pylori in patients with recurrent aphthous stomatitis. Oral Dis. 2005;11:22-26.
- Fox JG, Batchelder M, Marini R, et al. Helicobacter pylori induced gastritis in the domestic cat. Infect Immun. 1995;63:2674-2681.
- Ozdemir A, Mas MR, Sahin S, Sa?lamkaya U, Ate?kan U. Detection of Helicobacter pylori colonization in dental plaques and tongue scrapings of patients with chronic gastritis. Quintessence Int. 2001;32:131-134.
- Hoshi K, Yamano Y, Mitsunaga A, Shimizu S, Kagawa J, Ogiuchi H. Gastrointestinal diseases and halitosis: association of gastric Helicobacter pylori infection. Int Dent J. 2002;52(Suppl 3):207-211.
- Adler I, Denninghoff VC, Alvarez MI, Avagnina A, Yoshida R, Elsner B. Helicobacter pylori associated with glossitis and halitosis. Helicobacter. 2005;10:312-317.
- Shimoyama T, Horie N, Kato T, Kaneko T, Komiyama K. Helicobacter pylori in oral ulcerations. J Oral Sci. 2000;4:225-229.
- Shankaran K, Desai HG. Helicobacter pylori in dental plaque. J Clin Gastroenterol. 1995;21:82-84.
- Song Q, Lange T, Spahr A, Adler G, Bode G. Characteristic distribution pattern of Helicobacter pylori in dental plaque and saliva detected with nested PCR . J Med Microbiol. 2000;49:349-353.
- Kamat AH, Mehta PR, Natu AA, et al. Dental plaque: an unlikely reservoir of Helicobacter pylori. Indian J Gastroenterol. 1998;17:138-140.
- Santamaria MJ, Varea Calderón V, Muñoz Almagro MC. Dental plaque in Helicobacter pylori infection. An Esp Pediatr. 1999;50:244-246.
- Savoldi E, Marinone MG, Negrini R, Facchinetti D, Lanzini A, Sapelli PL. Absence of Helicobacter pylori in dental plaque determined by immunoperoxidase. Helicobacter. 1998;3:283-287.
- Mattana CM, Vega AE, Flores G, de Domeniconi AG, de Centorbi ON. Isoloation of Helicobacter pylori from dental plaque. Rev Argent Microbiol. 1998;30:93-95.
- Majmudar P, Shah SM, Dhunjibhoy KR, Desai HG. Isolation of Helicobacter pylori from dental plaques in healthy volunteers. Indian J Gastroenterol. 1990;9:271-272.
- Avcu N, Avcu F, Beyan C, et al. The relationship between gastric-oral Helicobacter pylori and oral hygiene in patients with vitamin B12-deficiency anemia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:166-169.
- Song Q, Spahr A, Schmid RM, Adler G, Bode G. Helicobacter pylori in the oral cavity: high prevalence and great DNA diversity. Dig Dis Sci. 2000;45:2162-2167.
- Vaira D, Holton J, Cairns S, et al. Urease test for Campylobacter pylori: care in interpretation. J Clin Pathol. 1988;41:812-813.
- Nguyen AM, Engstrand L, Genta RM, Graham DY, el-Zaatari FA. Detection of Helicobacter pylori in dental plaque by reverse transcription-polymerase chain reaction. J Clin Microbiol. 1993;31:783-787.
- Gebara EC, Pannuti C, Faria CM, Chehter L, Mayer MP, Lima LA. Prevalence of Helicobacter pylori detected by polymerase chain reaction in the oral cavity of periodontitis patients. Oral Microbiol Immunol. 2004;4:277-280.
- Young KA, Allaker RP, Hardie JM. Morphological analysis of Helicobacter pylori from gastric biopsies and dental plaque by scanning electron microscopy. Oral Microbiol Immunol. 2001;16:178-181.
- Umeda M, Kobayashi H, Takeuchi Y, et al. High prevalence of Helicobacter pylori detected by PCR in the oral cavities of periodontitis patients. J Periodontol. 2003;74:129-134.
- Gebara EC, Faria CM, Pannuti C, Chehter L, Mayer MP, Lima LA. Persistence of Helicobacter pylori in the oral cavity after systemic eradication therapy. J Clin Periodontol. 2006;33:329-333.
- Hu W, Cao C, Meng H. Helicobacter pylori in dental plaque of periodontitis and gastric disease patients. Zhonghua Kou Qiang Yi Xue Za Zhi. 1999;34:49-51.
- Doré-Davin C, Heitz M, Yang H, Herranz M, Blum AL, Corthésy-Theulaz I. Helicobacter pylori in the oral cavity reflects handling of contaminants but not gastric infection. Digestion. 1999;60:196-202.
- Kignel S, de Almeida Pina F, André EA, Alves Mayer MP, Birman EG. Occurrence of Helicobacter pylori in dental plaque and saliva of dyspeptic patients. Oral Dis. 2005;11:17-21.
- Okuda K, Ishihara K, Miura T, Katakura A, Noma H, Ebihara Y. Helicobacter pylori may have only a transient presence in the oral cavity and on the surface of oral cancer. Microbiol Immunol. 2000;44:385-388.
- Riggio MP, Lennon A, Wray D. Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. J Oral Pathol Med. 2000;29:507-513.
- Parsonnet J, Shmuely H, Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA. 1999;282:2240-2245.
- Souto R, Colombo AP. Detection of Helicobacter pylori by polymerase chain reaction in the subgingival biofilm and saliva of non-dyspeptic periodontal patients. J Periodontol. 2008;79:97-103.
- Berroteran A, Perrone M, Correnti M, et al. Detection of Helicobacter pylori DNA in the oral cavity and gastroduodenal system of a Venezuelan population. J Med Microbiol. 2002;51:764-770.
- Asikainen S, Chen C, Slots J. Absence of Helicobacter pylori in subgingival samples determined by polymerase chain reaction. Oral Microbiol Immunol. 1994;9:318-320.
- Martinez-Gomis J, Diouf A, Lakhssassi N, Sixou M. Absence of Helicobacter pylori in the oral cavity of 10 non-dyspeptic subjects demonstrated by real-time polymerase chain reaction. Oral Microbiol Immunol. 2006;21:407-410.
- Miyabayashi H, Furihata K, Shimizu T, Ueno I, Akamatsu T. Influence of oral Helicobacter pylori on the success of eradication therapy against gastric Helicobacter pylori. Helicobacter. 2000;5:30-37.
- Costerton JW, Cheng K, Geesey GG, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435-439.
- Chitsazi MT, Fattahi E, Farahani RMZ, Fattahi S. Helicobacter pylori in the dental plaque: is it of diagnostic value for gastric infection? Med Oral Patol Oral Cir Bucal. 2006;11:E325-E328.
- Sahin FI, Tinaz AC, Simsek IS, Menev?e S, Görgül A. Detection of Helicobacter pylori in dental plaque and gastric biopsy samples of Turkish patients by PCR-RFLP. Acta Gastroenterol Belg. 2001;64:150-152.
- Cammarota G, Tursi A, Montalto M, et al. Role of dental plaque in the transmission of Helicobacter pylori infection. J Clin Gastroenterol. 1996;22:174-177.
- Cheng LH, Webberley M, Evans M, Hanson N, Brown R. Helicobacter pylori in dental plaque and gastric mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:421-423.
- Hardo PG, Tugnait A, Hassan F, et al. Helicobacter pylori infection and dental care. Gut. 1995;37:44-46.
- Butt AK, Khan AA, Khan AA, et al. Correlation of Helicobacter pylori in dental plaque and gastric mucosa of dyspeptic patients. J Pak Med Assoc. 2002;52:196-200.
- Cellini L, Allocati N, Piattelli A, Petrelli I, Fanci P, Dainelli B. Microbiological evidence of Helicobacter pylori from dental plaque in dyspeptic patients. New Microbiol. 1995;18:187-192.
- Shames B, Krajden S, Fuskja M, Babida C, Penner JL. Evidence for the occurrence of the same strain of Campylobacter pylori in the stomach and dental plaque. J Clin Microbiol. 1989;27:2849-2850.
- Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biologic sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol. 1995;15:56-63.
- Dowsett SA, Archila L, Segreto VA, et al. Helicobacter pylori infection in indigenous families of Central America: serostatus and oral and fingernail carriage. J Clin Microbiol. 1999;37:2456-2460.
- Robinson LG, Black FL, Lee FK, et al. Helicobacter pylori prevalence among indigenous peoples of South America. J Infect Dis. 2002;186:1131-1137
From Dimensions of Dental Hygiene. May;6(5): 16-21.