
Exploring the Relationship
A closer look at the association between periodontal disease and rheumatoid arthritis.
This course was published in the March 2008 issue and expires March 2011. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the immunological, biological, and genetic associations between rheumatoid arthritis and periodontal disease.
- Describe evidence to support the theory that certain common bacteria may be etiologically linked to rheumatoid arthritis and periodontal diseases.
- Describe the evidence that suggests that the severity of rheumatoid arthritis and the severity of periodontal disease may be interrelated.
- Define the evidence that suggests that periodontal treatment may have an effect on rheumatoid arthritis.
While I was working in a practice that treated a large geriatric population about 10 years ago, I noticed a higher incidence of periodontal disease in patients with rheumatoid arthritis (RA). Over time, I also observed that with treatment of the periodontitis, many reported a decrease in the joint pain associated with RA following scaling and root planing procedures. Little did I know that a growing body of evidence supports a relationship between periodontal disease and RA.
Although the proposed relationship between RA and periodontal disease is somewhat controversial, a number of published works1-5 over the past decade have reported a significant association between the extent and severity of periodontal disease and RA. This relationship may be explained by an underlying dysfunction of the basic inflammatory mechanisms common to both diseases.
Both diseases result from an imbalance between pro-inflammatory and anti-inflammatory cytokines (small secreted proteins that mediate and regulate immunity, inflammation, and hematopoiesis). So many similarities exist between these two inflammatory conditions that some researchers suggest the two are really part of the same disease.6 However, not all findings are in agreement. Differences in disease criteria and the methodology used to evaluate periodontal status have complicated the interpretation of data on the subject.7
RA is a chronic inflammatory multisystem autoimmune disease affecting 1% of the adult population. Its symptoms include destruction of joint cartilage and bone, joint pain, and decreased mobility.8 RA may be initiated by an exposure event (either microbial challenge or autoantigen) that leads to significant inflammation and tissue destruction.9 As a systemic disease, RA is manifested in systems such as the pulmonary, ocular, and vascular systems, and other organs that may be affected by the inflammatory process. RA is a localized condition with systemic sequela, much like periodontal disease.9
Periodontal disease, one of the most common diseases in humans, affects 10%-15% of all adults and roughly one third of all adults beyond the fifth decade of life.8 It is considered the leading cause of tooth loss in adults. Periodontal infection is initiated by gram negative anaerobic bacteria that lead to the progression and destruction of supporting structures surrounding the tooth.8 Great variability exists in host response to the challenge of gram negative anaerobic bacteria, which is influenced by systemic, environmental, and genetic risk factors, all of which impact susceptibility and the presentation of periodontal disease.
SHARED FEATURES
RA and periodontal disease are chronic inflammatory diseases that have similar immunological, biological, and genetic features. Figure 1 describes many of the similarities between these two diseases. Both are characterized by local destruction/degradation of hard and soft tissues (connective tissue and bone) due to persistent inflammation.7,10,11
There is an increased probability that the two diseases co-exist.2,5,12 A variety of cytokines and matrix metalloproteinases (MMPs) are upregulated and intimately involved in the pathogenesis of both periodontal disease and RA. These effector molecules are common to both diseases.9 The coexistence of RA and periodontal disease seems to not affect clinical manifestations of periodontal disease or systemic markers of RA. Some researchers have concluded that an altered systemic immune response may be shared between patients with periodontal disease and chronic arthritis, distinguishing them from disease-free individuals.13
More than 20 years of evidence has established that pro- and anti-inflammatory cytokines are common features of periodontal disease,14-16 juvenile idiopathic arthritis (JIA),17-19 and RA.20-23 Similarities in periodontal and hematological variables have been observed in individuals with aggressive periodontitis, JIA, and RA, as compared to controls.13 In RA and JIA, both of which are autoimmune diseases, traditional markers of inflammation reflect disease activity and response to treatment, which are demonstrated by raised levels of blood cell counts and acute phase reactants.13 Periodontal disease is also related to similar elevated markers and to the presence of periodontal pathogens.13,24 Evidence supports that gingival crevicular fluid and periodontal tissues of inflamed sites are rich in cytokines,25-27 which are similar to the cytokines found in the inflamed synovial membrane and fluid in individuals with arthritis.17,20,28
Investigations into the potential for a genetic link between RA and periodontal disease continue. In a recent study population of 100 Japanese adults with RA, 100 with periodontitis only, and 100 healthy controls, Kobayashi and colleagues29 reported that although IL-1 and Fc? gene polymorphisms (genes that appear in several forms related to regulation of immune responses usually associated with infection and inflammation) did not appear to be common risk factors for RA and periodontitis, distributions of certain related genotypes and halotypes may be unique to patients with RA and periodontal disease. The researchers proposed that the IL-1 combined risk alleles (alternative forms of genes that may occur at a given position in a chromosome) may cause a synergistic effect on bone destruction in joints and the periodontium, which leads to increased susceptibility to both diseases.
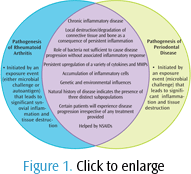 The natural histories of RA and periodontal disease bear remarkable similarities. Each disease is divided into three subpopulations, which are categorized according to disease extent and severity (Table 1).
The natural histories of RA and periodontal disease bear remarkable similarities. Each disease is divided into three subpopulations, which are categorized according to disease extent and severity (Table 1).
IS BACTERIA THE LINK?
The concept that ongoing periodontal disease could trigger RA in genetically susceptible individuals is plausible.9 However, this remains speculative until causative agents for RA can be definitively identified. There may be no single primary cause of RA; it may be that different mechanisms independently lead to synovial inflammation in a susceptible individual.9
A recently published animal study adds further validity of a relationship between periodontitis and RA. Ramamurthy and colleagues34 reported that induced arthritis in rats resulted in periodontal destruction noted by alveolar bone loss and increased MMP activity in the rats’ gingival tissues. These events occurred without manipulating the oral or subgingival microflora, suggesting that bacterial burden was not a factor in the periodontal destruction.
|
|||||||||||||||
RA DRUGS AND PERIO STATUS
Despite intervention with anti-inflammatory/anti-rheumatoid drug regimens, and similar levels of plaque and periodontal inflammation, Havemose-Poulsen and colleagues13 found that young adults (≤35 years) with RA possess a significantly higher percentage of sites with pocket depth ≥4 mm, CAL ≥ 2mm, and alveolar bone loss ≥ 2 mm, compared with healthy individuals. They also found that in patients with RA, the percentage of sites with pocket depths ≥ 4 mm was significantly correlated to the percentage of sites with bleeding on probing, CAL ≥ 2 mm, and plaque. The percentage of sites with CAL ≥ 2 mm was significantly correlated with levels of the antibodies IgA-RF and IgM-RF (immunoglobulins that include antibodies related to the immune response associated with rheumatoid arthritis).
In contrast, another study35 analyzed markers of periodontal inflammation, IL-1β and -18, and elastase activity in gingival crevicular fluid of individuals with RA and compared them to controls matched for major confounders.35 Total amounts of IL-1β and elastase activity (the activity of an enzyme that digests elastin, a protein similar to collagen, which is the main component of elastic fibers) were significantly lower in the RA individuals. Investigators proposed that intensive, systemic anti-inflammatory therapy aimed at reducing RA disease activity, (ie, retarding joint erosion and improving patients’ quality of life), probably interferes with destructive processes in the periodontium as well.
In a study of 23 RA patients, 17 systemically healthy patients with periodontal disease, and 17 systemically and periodontally healthy patients, Biyikoglu and colleagues36 concluded that the similarity in GCF levels of PGE2 and IL-1β in RA and periodontal disease groups—despite the long term use of corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs)—showed that RA patients may have a propensity to overexpress inflammatory mediators. They speculated that these immunosuppressive drugs and NSAIDs suppress the production of inflammatory mediators in RA patients. The researchers further concluded that without long-term NSAID suppression, RA patients would exhibit higher amounts of these mediators that could possibly lead to increased periodontal destruction.
LINKED BY SEVERITY?
Although the etiologies of RA and periodontal disease are distinctly separate, the underlying pathological processes are so similar that Mercado and colleagues5 investigated whether individuals at risk of developing RA may also be at risk of developing periodontitis, and/or vice versa. The researchers hypothesized that if individuals have a particular systemic predisposition toward altered immune function or altered connective tissue metabolism, they may also be susceptible to multiple diseases related to these underlying dysfunctions.
To test this theory, the study assessed 1,412 individuals for the prevalence of periodontal disease and RA, with the primary goal of determining whether individuals with periodontal disease would have a higher prevalence of RA than those without periodontitis. The secondary goal was to see if individuals with RA would have a higher prevalence of advanced forms of periodontal destruction than patients with periodontal disease but without RA. The data showed that in patients referred for periodontal treatment, the prevalence of self-reported RA was 3.95%. This is significantly higher than in patients who were not referred for periodontal treatment (0.66%) and the percentage of self-reported RA in the general population (1%). Further, 62.5% of those referred patients with RA had advanced forms of periodontal disease. Overall, the prevalence of moderate to severe periodontitis was significantly higher in individuals with RA. Patients with periodontitis also had a higher prevalence of RA compared to the general population. These findings compelled the investigators of this research5 to propose that “in a proportion of RA patients with moderate-to-severe periodontitis, an unidentified disablement or disregulation of common pathologic mechanisms operate in these chronic inflammatory diseases.”
In a follow-up study,1 the same researchers looked at whether individuals with RA would be more likely to experience periodontal disease and if the incidence of severe periodontitis would be higher in these patients. A population of 65 patients with RA was studied to determine the extent of periodontal disease. These findings were then correlated with various indicators of RA.
The control group included age and gender-matched individuals who did not have RA. Although there was no difference in the plaque and bleeding indices between the control and RA groups, the RA group had significantly more missing teeth and a greater percentage of deeper periodontal pockets compared to the control group. In addition, the percentage of alveolar bone loss correlated positively with RA severity. This finding helps dispel the notion that RA patients have more plaque deposits because of limited dexterity imposed by their RA and suggests that the amount of destruction seen in the RA group is relative to the inflammatory response. Secondly, Mercado and colleagues1 concluded that RA patients are more than twice as likely to experience moderate-to-severe periodontal bone loss and pocket depths? 6.2 mm compared to those without RA.
EFFECT OF PERIO TREATMENT
 A study reported by Ribeiro and colleagues7 suggests that periodontal treatment with scaling and root planning (SRP) might have a significant effect on reducing the erythrocyte sedimentation rate (ESR), a nonspecific measure of inflammation. The reduction of ESR that accompanies SRP might be due to a decrease in infection and severity of periodontal disease. This cascade is initiated when the infection stimulates the production of globulins and plasmatic proteins such as haptoglobulins, C-reactive protein, fibrinogen and, by association, increases the levels of ESR. In the study, periodontal infection increased the fibrinogen levels that can be measured by ESR.7 After periodontal treatment with SRP, the degree of severe incapacity from RA was decreased.7
A study reported by Ribeiro and colleagues7 suggests that periodontal treatment with scaling and root planning (SRP) might have a significant effect on reducing the erythrocyte sedimentation rate (ESR), a nonspecific measure of inflammation. The reduction of ESR that accompanies SRP might be due to a decrease in infection and severity of periodontal disease. This cascade is initiated when the infection stimulates the production of globulins and plasmatic proteins such as haptoglobulins, C-reactive protein, fibrinogen and, by association, increases the levels of ESR. In the study, periodontal infection increased the fibrinogen levels that can be measured by ESR.7 After periodontal treatment with SRP, the degree of severe incapacity from RA was decreased.7
The results of another study mirror these findings. Al-Katmna and colleagues37 investigated whether eliminating periodontal infection and gingival inflammation affects the severity of active RA in a small group (29) of patients with chronic inflammatory periodontal disease. Their findings suggest that control of periodontal infection and gingival inflammation by SRP and plaque control in subjects with moderate periodontal disease may contribute to a reduction in the signs and symptoms of active RA. Patients in the treatment group reported a subjective improvement in their arthritic condition. The investigators theorized that the improvement in the treatment group might be due to a decrease in the inflammatory products in the blood after periodontal therapy. Another explanation is that the elimination of bacterial plaque and endotoxins as a result of periodontal treatment may also contribute to improving RA by reducing exposure of the joint structures to bacteria and their products.
EXAMINING INFLAMMATION
Recent data from the study8 of 100 active RA patients demonstrated not only an association between RA, periodontal disease, and coronary artery disease (CAD), but also the association of inflammatory, lipid, and hemostatic markers of these diseases. The RA patients, 50% of whom had established CAD, and 50% of whom had no CAD, were all assessed for periodontal disease. The RA patients with CAD had significantly more periodontal disease than RA patients without. Inflammatory markers were elevated in all patients but were significantly higher in RA patients with CAD who also had periodontal disease. These findings suggest that patients with all three of these diseases have more inflammatory disease burden, and that systemic inflammation perhaps confers additional risk for cardiovascular disease in RA patients. The research supports the idea that chronic inflammation, periodontal disease in particular, is a risk factor for cardiovascular disease.
PRACTICAL APPLICATION
The likelihood that periodontal disease is associated with various systemic diseases and conditions such as coronary heart disease and stroke; complications of diabetes; and adverse pregnancy outcomes, is becoming established fact. Although some controversy remains over the true nature of the association between RA and periodontal disease, emerging evidence makes it difficult to deny the inter-relationship that may exist. However, this area of investigation needs further research. Nevertheless, implementing some of the evidence discussed above in clinical practice is possible.
- A certain group of individuals seem to have increased susceptibility to severe periodontal disease. For this reason, it is important that systemic and local factors related to increased severity of periodontal disease be identified. This allows for interception and early treatment of those in this high risk category. A recent study38 that examined the association between risk indicators for periodontal disease severity and tooth loss due to periodontal reasons found that RA was one of the strongest risk indicators for periodontitis-induced tooth loss. RA should be included as a risk factor when performing a periodontal evaluation. (See table on odds ratio).
- The periodontal status of patients with established RA should be carefully monitored and recorded.9
- Young adults (?35 years) with RA may develop periodontal destruction early in the course of arthritic disease.13 These patients require early intervention. To improve the dental health of RA patients, prophylactic programs may be beneficial as the severity of periodontal disease (as measured by the percentage of sites ? 4mm) in RA patients appears to correlate with the percentage of sites with plaque and bleeding on probing. Programs designed to prevent periodontal complications in these patients are recommended.13
- Abou-Raya and colleagues8 found that inflammation may be the central link between chronic inflammatory and autoimmune disorders such as RA, periodontal disease, and atherosclerosis. Accordingly, early identification of risk factors and intervention for periodontal disease are necessary. This disease management strategy allows for “effective dampening of the inflammatory activity,” which may translate into lessening the risk of cardiovascular disease and mortality in RA patients.
- Watch for new treatment strategies that inhibit pro-inflammatory cytokines and destructive proteases, which are expected to emerge for treatment of both diseases.9
REFERENCES
- Mercado FB, Marshall RI, Klestov AC, et al. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779-787.
- Kässer UR, Gleissner C, Dehne F, et al. Risk for periodontal disease in patients with longstanding rheumatoid arthritis. Arthritis Rheum. 1997;40:228-2251.
- Gleissner C, Willershausen B, Kaesser U, et al. The role of risk factors for periodontal disease in patients with rheumatoid arthritis. Eur J Med Res. 1998;3:387-392.
- Albandar JM. Some predictors of radiographic alveolar bone height reduction over 6 years. J Periodontal Res. 1990;25:186-192.
- Mercado F, Marshall RI, Klestov AC, et al. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol. 2000;27:267-272.
- Greenwald RA, Kirkwood K. Adult periodontitis as a model for rheumatoid arthritis with emphasis on treatment strategies. J Rheumatol. 1999;26:1650-1653.
- Ribeiro J, Leão A, Novaes A. Periodontal infection as a possible severity factor for rheumatoid arthritis. J Clin Periodontol. 2005;32:412-416.
- Abou-Raya S, Abou-Raya A, Naim A, et al. Rheumatoid arthritis, periodontal disease and coronary artery disease. Clin Rheumatol. 2007;Aug 29.
- Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: A review. J Periodontol. 2005;76:2066-2074.
- Grassi W, De Angelis R, Lamanna G, et al. The clinical features of rheumatoid arthritis. Eur J Radiol. 1998;27(Suppl 1):S18-24.
- Mercado FB, Marshall RI, Bartold PM. Inter-relationships between rheumatoid arthritis and periodontal disease. A review. J Clin Periodontol. 2003;30:761-772.
- Miranda LA, Fischer RG, Sztajnbok FR, et al. Periodontal conditions in patients with juvenile idiopathic arthritis. J Clin Periodontol. 2003;30:969-974.
- Havemose-Poulsen A, Westergaard J, Stoltze K, et al. Periodontal and hematological characteristics associated with aggressive periodontitis, juvenile idiopathic arthritis and rheumatoid arthritis. J Periodontol. 2006;77:280-288.
- Kjeldsen M, Holmstrup P, Bendtzen K. Marginal periodontitis and cytokines: A review of the literature. J Periodontol. 1993;64:1013-1022.
- Havemose-Poulsen A, Holmstrup P. Factors affecting Il-1-mediated collagen metabolism by fibroblasts and the pathogenesis of periodontal disease: A review of the literature. Crit Rev Oral Biol Med. 1997;8:217- 236.
- Graves DT, Cochran D. The contri bution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391401.
- Mangge H, Schauenstein K. Cytokines in juvenile rheumatoid arthritis. Cytokine. 1998;10:471-480.
- Woo P. Cytokines in juvenile chronic arthritis. Baillieres Clin Rheumatol. 1998;12:219-228.
- Müller K, Herner EB, Stagg A, et al. Inflammatory cytokines and cytokine anatagonists in whole blood cultures of [patients with systemic juvenile chronic arthritis. Br J Rheumatol. 1998;37:562-569.
- Feldman M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397-440.
- Bingham CO III. The pathogenesis of rheumatoid arthritis: Pivotal cytokines involved in bone degradation and inflammation. J Rheumatol. 2002;65(Suppl):3-9.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356-361.
- Dayer JM. The process of identifying and understanding cytokines: from basic studies to treating rheumatoid diseases. Best Pract Res Clin Rheumatol. 2004;18:31-45.
- Noack B, Genco RJ, Trevisan M, et al. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221-1227.
- Champagne CM, Buchanan W, Reddy MS, et al. Potential for gingival crevice fluid measures as predictors for risk for periodontal diseases. Periodontol 2000. 2003;31:167-180.|
- Roberts FA, Hockett RD Jr, Bucy RP, et al. Quantitative assessment of inflammatory cytokine gene expression in chronic periodontitis. Oral Microbiol Immunol. 1997;12:336-344.
- Gemmell E, Seymour GC. Cytokine profiles of cells extracted from humans with periodontal diseases. J Dent Res. 1998;77:16-26.
- Vervoordeldonk MJ, Tak PP. Cytokines in rheumatoid arthritis. Curr Rheumatol Rep. 2002;4:208-217.
- Kobayashi T, Ito S, Kuroda T, et al. The Interleukin-1 and Fc? receptor gene polymorphisms in Japanese patients with rheumatoid arthritis and periodontitis. J Periodontol. 2007;78:2311-2318.
- Hirschfeld L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49:225-237.
- O’Sullivan JB, Cathcart ES. The prevalence of rheumatoid arthritis. Follow-up evaluation of the effect of criteria on rates in Sudbury, Massachusetts. Ann Intern Med. 1972;76:573-577.
- Pincus T, Marcum SB, Callahan LF. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheumatolog. 1992;19:1874-1884.
- Wolfe F, Hawley DJ, Cathey MA. Termination of slow acting antirheumatic therapy in rheumatoid arthritis: a 14 year prospective evaluation of 1017 consecutive starts. J Rheumatolog 1990;17:994-1002.
- Ramamurthy NS, Greenwald RA, Celiker MY, et al. Experimental arthritis in rats induces biomarkers of periodontitis which are ameliorated by gene therapy with tissue inhibitor of matrix metallo proteinases. J Periodontol. 2005;76:229-233.
- Miranda LA, Islabão AG, Fischer RG, et al. Decreased interleukin-1_ and elastase in the gingival crevicular fluid of individuals undergoing anti-inflammatory treatment for rheumatoid arthritis. J Periodontol. 2007;78:1612-1619.
- Biyikoglu B, Buduneli N, Kardesler L, et al. Evaluation of t-PA, PAI-2, IL-1beta and PGE2 in gingival crevicular fluid of rheumatoid arthritis patients with periodontal disease. J Clin Periodontol. 2006;33:605-611.
- Al-Katma MK, Bissada N, Bordeaux JM, et al. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatolog. 2007;13:134-137.
- Al-Shammari KF, Al-Khabbaz AK, Al-Ansari JM, et al. Risk indicators for tooth loss due to periodontal disease. J Periodontol. 2005;76:1910-1918.
From Dimensions of Dental Hygiene. March 2008;6(3): 30-33.



