 XIXINXING/ISTOCK/GETTY IMAGES PLUS
XIXINXING/ISTOCK/GETTY IMAGES PLUS
Dental Aerosols: The Infection Connection
Oral health professionals need to be aware of the risks posed by dental aerosols in order to reduce the risk of disease transmission before, during, and after patient care.
Respiratory infections—both chronic and acute—contributed to 6 million deaths in 2016, and they are highly communicable.1–3 Many respiratory infections have been linked to the microbial inhabitants of the oral cavity.4–10 Dental procedures that use low- and high-speed handpieces, laser or electrosurgery units, ultrasonic scalers, air polishers, prophy angles, hand instrumentation, and air/water syringes can create bioaerosols and spatter.9,11 Ultrasonic scalers and high-speed handpieces produce more airborne contamination than any other instrument in dentistry.9,12,13 Inhalation of airborne particles and aerosols produced during dental procedures may cause adverse respiratory health effects and bidirectional disease transmission.8,9,11,12,14–24 While there are recommended mechanisms that minimize the risk of transmission, many may not be routinely practiced.9,12,17,19,21 Oral health professionals need to be aware of the invisible dangers in the operatory and reduce the risk of disease transmission before, during, and after patient care.
ASSOCIATED RESPIRATORY INFECTIONS
The oral cavity is inhabited by more than 700 bacterial species. It harbors fungi and viruses from the respiratory tract.4,9,25 Temperature, pH, nutrients, host defenses, and host genetics contribute to microbial growth.26,27 Many normal inhabitants of the oral cavity include species of Streptococcus, Actinomyces, Neisseria, Campylobacter, Porphyromonas, Prevotella, Capnocytophaga, and Fusobacteria.5,21,26 The oral microenvironment can be altered by factors such as age, tooth eruption or loss, oral disease status, pregnancy, and drug use. Several of these changes can alter the virulence and pathogenicity of microorganisms.27,28
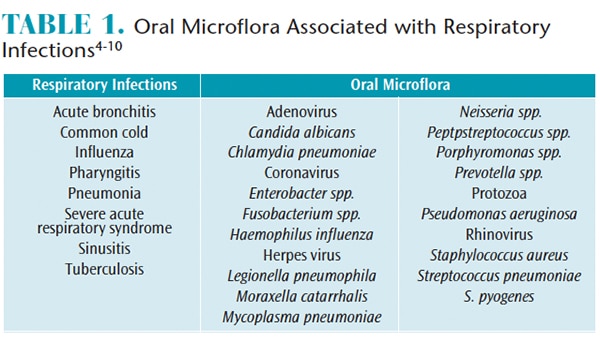
Although direct disease transmission has not been confirmed, oral microflora in healthy and diseased states has been linked to respiratory infections.6–9,29 Such infections include the common cold, sinusitis, pharyngitis, pneumonia, tuberculosis, severe acute respiratory syndrome (SARS), and influenza (Table 1).4–10 Furthermore, nasal congestion, headaches, and asthmatic episodes have also been triggered by dental bioaeresols.30
![Dental Aerosols]() AEROSOL PRODUCTION
AEROSOL PRODUCTION
Potential routes of infection in dental settings are direct and indirect contact, inhalation, and injections/punctures.9,13,31 These routes can be bidirectional where transmission may occur from patient-to-patient, patient-to-clinician, or clinician-to-patient.24 Inhalation of bioaerosols is considered a moderate risk for disease transmission.31 Dental patients and personnel are exposed to tens of thousands of bacteria per cubic meter, and the potential to breathe infective material that is aerosolized during routine dental procedures is highly likely.13,30 Due to the size, composition, and ability to linger, airborne particles pose an increased health risk.14,17,19,31
Aerosols are differentiated based on particle size: spatter (> 50 µm), droplet (≤ 50 µm), and droplet nuclei (≤ 10 µm).9,14,18,31 In dental settings, 90% of aerosols produced are extremely small (< 5 µm).18 Spatter, being the larger particle, will fall until it contacts other objects (floor, countertop, sinks, bracket, table, computer, patient, operators, etc).9,19 Droplets remain suspended in the air until they evaporate, leaving droplet nuclei that contain bacteria related to respiratory infections.9,14 Droplet nuclei can contaminate surfaces in a range of 3 feet and may remain airborne for 30 minutes to 2 hours.9,10,13,17,18,30 If inhaled, the droplet nuclei can penetrate deep into the respiratory system.9,14,17–19,31 Furthermore, the susceptibility of developing an infection is influenced by virulence, dose, and pathogenicity of the microorganisms and the host’s immune response.10
Oral biofilm is resistant to antibiotics, antimicrobial agents, and the body’s immune system; therefore, removal of bacteria in dental biofilms is best achieved by physical disruption, contributing to aerosol production.5,7 Aerosols, both visible and invisible, are created during the performance of surgical and nonsurgical dental therapies, which may include extractions, crown preparations, caries restorations, periodontal therapies, prophylaxes, and endodontic therapies.13,22,32 Aerosols consist of water, saliva, blood, debris, and microorganisms (bacteria, fungi, viruses, protozoa) and their metabolites, such as lipopolysaccharides/endotoxins and other toxins.11,30
Numerous studies have identified specific microbes aerosolized during dental procedures.21,22,33,34 Manarte-Monteiro et al33 determined species of Micrococcus, Staphylococcus, and Streptococcus were aerosolized during endodontic and restorative treatments. Rautemaa et al34 found the most common bacteria aerosolized during restorative treatment using high-speed instruments were viridans streptococci and staphylococci. Pina-Vaz et al22 discovered streptococci were aerosolized during endodontic access. Feres et al21 identified species of Actinomyces, Fusobacterium, Capnocytophaga, and Streptococcus that were aerosolized during periodontal therapy.
Dental unit waterlines have also been shown to harbor bacteria. The specific bacteria likely to cause diseases include Legionella, Pseudomonas, and Nontuberculous mycobacteria.13,35 Dental treatments and aerosolized bacteria from waterlines can cause serious infections.13,35 For example, in 2011, an 82-year-old woman died from Legionnaires’ disease contracted during dental treatment.36
AEROSOL PREVENTION AND REDUCTION
Many safety procedures should be incorporated into daily practice to minimize aerosol production, prevent contamination from emitted particles, and reduce transmission of infectious diseases. Minimizing dissemination of aerosols can be accomplished through the use of high velocity air evacuation and preprocedural antimicrobial mouthrinses, flushing waterlines at the beginning of the work day and between each patient, wearing personal protective equipment (PPE), and employing air purifications systems.9,11–13,22 These recommendations are outlined in the United States Centers for Disease Control and Prevention (CDC) Guidelines for Infection Control in Dental Health-Care Settings—2003.13 In 2016, the CDC published the Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care.35 This guide highlights the existing CDC recommendations, provides basic infection prevention principles and recommendations for oral health professionals, reaffirms standard precautions as the foundation for preventing transmission of infectious agents, and provides links to the full guidelines and source documents.35
The high-volume evacuator (HVE) has a large diameter (> 8 mm), which allows for removal of high volumes of air in a short time and reduces the amount of bioaerosols by as much as 90%.9,11–18 The HVE can be challenging to maneuver simultaneously with instrumentation; therefore, most dental hygienists find the saliva ejector easier to use, making it the preferred device for removing excess fluids from the oral cavity.9 From a practice and safety standpoint, it’s important to note that the opening size of the saliva ejector makes it inadequate for reducing aerosols compared with the HVE or an isolation and evacuation device.9,19,22,31
Although the CDC does not have specific recommendations for using preprocedural antimicrobial mouthrinses to reduce aerosol exposure, studies show that their use for 60 seconds significantly reduces the level of oral microorganisms in the aerosols generated during routine dental procedures.9,11,17,18,20
Water in the dental treatment setting should meet the US Environmental Protection Agency standards for drinking water (<500 CFU/mL of heterotrophic water bacteria).13,35 To ensure the delivery of quality water, manufacturer instructions for use (IFU) should be followed for the dental unit and any waterline treatment products.13,35 Monitoring water quality can be conducted by an outside laboratory or in-office with self-contained testing kits.13,35 Additionally, independent reservoirs, chemical treatment, filtration, sterile water delivery system, or combinations of technologies are methods of improving and maintaining water quality.13,35
The CDC recommends water and air should be discharged for a minimum of 20 seconds to 30 seconds after each patient.13,35 This should be completed for all devices that connect to a waterline and enter patient mouths, such as handpieces, ultrasonic scalers, and air/water syringes.13,35 Some IFU require purging at the beginning of the workday and between patients for 2 minutes; therefore, oral health professionals should follow the IFU specific to their devices. Additionally, the manufacturer advice for testing and maintenance of anti-retraction devices (prevents water/fluid backflow in a waterline) must be followed.13,35
Standard precautions, as outlined by the CDC, involve the use of personal protective equipment (PPE).13,35 Primary PPE includes donning proper fitting gloves and surgical masks, protective eyewear with solid side shields or face shield, and protective clothing/disposable gowns.13,35 PPE should be worn whenever there is a potential to encounter spray or spatter during patient care and while disinfecting the treatment area, as bioaerosols remain suspended for 30 minutes to 2 hours post treatment.9,10,13,17,18,30,35 Masks and gloves should be changed between every patient; moreover, all PPE should be changed if torn, wet, or visibly soiled.13,35 If providing care for patients with a known infectious disease, the National Institute for Occupational Safety and Health (NIOSH) requires the wearing of a NIOSH-certified particulate-filter respirator.13 To reduce disease transmission, all PPE must be removed prior to exiting the treatment area.13
Aerosol control in confined, poorly ventilated spaces where the air exchange with filtration cannot be successfully applied presents a challenge.15 Another hurdle is to decrease the indoor concentration of bioaerosols.15 While some indoor air purification techniques aim solely at aerosol concentration reduction, others are designed to inactivate viable bioaerosols.15 Strong evidence demonstrates ventilation in a practice setting can impact the spread of airborne infections.37
Air cleaning systems—such as high efficiency particulate air (HEPA) filters, gas filter cartridges, and electrostatic filters—assist in purifying the air in and outside of dental operatories.11 The HEPA filter systems direct air through a series of prefilters, which help to continuously catch airborne microorganisms and retain particles as small as 0.3 µm.11 The four-cylinder gas filter cartridge systems aid in reducing gases and vapors, including mercury, formaldehyde, and glutaraldehyde.11 The electrostatically charged post-filters/ion emitters aid in purification by reducing dust, particulates, and vapors.11,16,38 Studies show that all three systems have been successful in significantly reducing bioaerosols created during dental procedures, most notably during cavity preparations, tooth extractions, and ultrasonic use.15,38,39
Ultraviolet germicidal irradiation (UVGI) units, commonly employed in hospital operatories and waiting areas, are successful adjunctive means that aid in eliminating aerosols.39 The high spectral emission lamps from these units produce photons that expose microorganisms to a short wavelength (254 nm) that deactivates DNA.17,19,39 This wavelength is lethal to a variety of microorganisms, especially Mycobacterium tuberculosis and Escherichia coli.17,19,39
CONCLUSION
Patients and oral health professionals are regularly exposed to tens of thousands of aerosols generated during dental procedures.30 This exposure increases the risk of respiratory infections.24,30 To ensure safety and reduce risk of respiratory infections, oral health professionals should abide by current CDC guidelines and recommendations. This includes using HVE, isolation and evacuation systems, and preprocedural mouthrinses, maintaining dental unit water quality, and wearing proper PPE.30 To further reduce risks and improve air quality, air cleaning systems can be employed.11,12,15–17,21,37–39 Combining multiple methods may be the most effective approach.
REFERENCES
- World Health Organization. The Top 10 Causes of Death. Available at: who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed September 28, 2018.
- GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1133–1161.
- Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66:1–75
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721-5732.
- Avila M, Ojcius DM, Yilmaz Ö. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–411.
- Brook I. Microbiology of sinusitis. Proc Am Thorac Soc. 2011;8:90–100.
- Gomes-Filho, IS, Passos JS, Seixas da Cruz S. Respiratory disease and the role of oral bacteria. J Oral Microbiol. 2010;2:5811.
- Scannapieco F. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–801.
- Harrel SK, Molinari J. Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–437.
- Zemori C, de Soet H, Criellard W, Laheij A. A scoping review on bioaerosols in healthcare and the dental environment. PLoS ONE. 2017;12:eo178007.
- Hallier C, Williams DW, Potts AJC, Lewis MAO. A pilot study of bioaerosol reduction using an air cleaning system during dental procedures. Br Dent J. 2010;209(8):E14.
- Rao RM, Shenoy N, Shetty V. Determination of efficacy of pre-procedural mouth rinsing in reducing aerosol contamination produced by ultrasonic scalers. Nitte University JHS. 2015;5:52–56.
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(No. RR-17).
- Atkinson J, Chartier Y, Pessoa-Silva CL, et al. Natural Ventilation for Infection Control in Health-Care Settings. Geneva: World Health Organization; 2009.
- Grinshpun SA, Adhikari A, Honda T, et al. Control of aerosol contaminants in indoor air: Combining the particle concentration reduction with microbial inactivation. Environ Sci Technol. 2007;41:606–612.
- Lee BU, Yermakov M, Grinshpun SA. Removal of fine and ultrafine particles from indoor air environments by the unipolar ion emission. Atmos Environ. 2004;38:4815–4823.
- Veena HR, Mahantesha S, Joseph PA, Patil SR, Patil SH. Dissemination of aerosol and splatter during ultrasonic scaling: A pilot study. J Infect Public Health. 2015; 8:260–265.
- James R, Mani A. Dental aerosols: A silent hazard in dentistry! Int J Sci Res. 2016;5:1761–1763.
- Szymanska J. Dental bioaerosol as an occupational hazard in a dentist’s workplace. Ann Agric Environ Med. 2007;14:203–207.
- Thomas MV, Jarboe G, Frazer RQ. Infection control in the dental office. Dent Clin N Am. 2008;52:609–628.
- Feres M, Figueiredo LC, Faveri M, Stewart B, de Vizio W. The effectiveness of a preprocedural mouthrinse containing cetylpyridinium chloride in reducing bacteria in the dental office. J Am Dent Assoc. 2010;141:415–422.
- Pina-Vaz I, Pina-Vaz C, de Carvalho MF, Azevedo A. Evaluating spatter and aerosol contamination during opening of access cavities in endodontics. Rev Clin Pesq Odontol. 2008;4(2):77-83.
- Polednik B. Aerosol and bioaerosol particles in a dental office. Environ Res. 2014;134:405–409.
- Laheij AMGA, Kistler JO, Belibasakis GN, Välimaa H, de Soet JJ. Healthcare-associated viral and bacterial infections in dentistry. J Oral Microbiol. 2012;4.
- Scannapieco F. The oral environment. In Lamont R, Hajishengalis G, Jenkinson H, eds. Oral Microbiology and Immunology. 2nd ed. Washington, DC: ASM Press; 2014:51–76.
- Sowmya Y. A review on the human oral microflora. J Dent Sci. 2016;9:1–5.
- Aas JA, Griffen AL, Dardis SR, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–1417.
- Najjar T. Bacterial mouth infections. Medscapes. Available at: emedicine.medscape.com/article/1081424-overview. Updated September 26, 2017. Accessed September 28, 2018.
- Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13:508–512.
- Dutil S, Meriaux A, de Latremoille MC, Lazure L, Barbeau J, Duchaine C. Measurement of airborne bacteria and endotoxin generated during dental cleaning. J Occup Environ Hyg. 2009;6:121–130.
- Sawhney A, Venugopal S, Babu G, et al. Aerosols how dangerous they are in clinical practice. J Clin Diagn Res. 2015;9:52–57.
- Yamada H, Ishihama K, Yasuda K, Hasumi-Nakayama Y, Shimoji S, Furusawa K. Aerial dispersal of blood-contaminated aerosols during dental procedures. Quintessence Int. 2011;42:399–405.
- Manarte-Monteiro P, Carvalho A, Pina C, Oliveira H, Manso MC. Air quality assessment during dental practice: Aerosols bacterial counts in an university clinic. Rev Port Estomatol Med Dent Cir Maxilofac. 2013;54:2–7.
- Rautemaa R, Nordberg A, Wuolijoki-Saaristo K, Meurman JH. Bacterial aerosols in dental practice: a potential hospital infection problem? J Hosp Infect. 2006;4:76–81.
- Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Atlanta: Centers for Disease Control and Prevention; October 2016.
- Ricci ML, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit waterline. Lancet. 2012;379:684.
- Li Y, Leung GM, Tang JW, et al. Role of ventilation in airborne transmission of infectious agents in the built environment: a multidisciplinary systematic review. Indoor Air. 2007;17:2–18.
- Martin SB, Moyer ES. Electrostatic respirator filter media: filter efficiency and most penetrating particle size effects. Appl Occup Environ Hyg. 2000;15:609–617.
- Noakes CJ, Fletcher LA, Beggs CB, Sleigh PA, Kerr KG. Development of a numerical model to simulate the biological inactivation of airborne microorganisms in the presence of ultraviolet light. J Aerosol Sci. 2004;35:489–507.
From Dimensions of Dental Hygiene. October 2018;16(10):12,14,16–17.