
Adverse Pregnancy Outcomes and Periodontal Diseases
This review investigates the evidence on the association between a variety of maternal health problems and the presence of periodontal pathogens.
This course was published in the December 2014 issue and expires December 31, 2017. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define adverse pregnancy outcomes.
- Discuss the global public health burden of low-birth weight, preterm birth, preeclampsia, and miscarriage/still births.
- Explain the role of periodontal diseases in adverse pregnancy outcomes.
- Detail the role of dental professionals in reducing the risk of low-birth weight, preterm birth, preeclampsia, and miscarriage/still births.
INTRODUCTION
We are fortunate to live in a country where significant medical advances occur on an almost daily basis. However, preterm birth is the second most common global cause of death in children younger than 5, and its prevalence seems to be increasing, despite considerable improvements in prenatal care and investment in research. One of the common risk factors for adverse pregnancy outcome is infection. Clearly the mouth—periodontal tissues in particular—represents a site of potential infection. Recently, increased attention has been paid to the role that periodontal diseases may play in adverse pregnancy outcomes. This review investigates the evidence for the association between various maternal health problems and the presence of periodontal pathogens.
The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support this article in collaboration with the American Academy of Periodontology. I hope you find this piece to be helpful in supporting the health of pregnant women in your practice.
—Barbara Shearer, BDS, MDS, PhD
Director of Scientific Affairs
Colgate Oral Pharmaceuticals
FROM THE AMERICAN ACADEMY OF PERIODONTOLOGY
As health care providers, we know that gender can play a significant role in treatment decisions. As oral health professionals, we understand that periodontal health is no different. While prevalence data from the United States Centers for Disease Control and Prevention and the American Academy of Periodontology (AAP) indicate that only 38% of women have periodontal diseases compared to 56% of men, the influence of periodontal diseases on women’s health changes throughout the stages of their lives—from puberty to menopause. The most studied aspect of women’s health and periodontal diseases is their impact on pregnancy outcomes. A large body of research indicates that pregnant women with periodontal diseases are more likely to deliver a preterm or low-birth weight baby. Evidence also shows that physiologic changes during pregnancy can affect a woman’s periodontal health—leading to conditions, such as pregnancy gingivitis, benign oral gingival lesions, loose teeth, tooth erosion, and even periodontitis. While debate in this area remains, sufficient evidence exists demonstrating that periodontal treatment during pregnancy may reduce the risk of adverse pregnancy outcomes. Dental hygienists are on the frontlines of detecting potential periodontal issues in pregnant patients and those of childbearing age, and of helping to determine the appropriate course of therapy. The establishment of a solid oral health regimen, paired with periodontal treatment recommendations from the patient’s dental professional and/or obstetrician, may help prevent negative outcomes. Early prevention and intervention are key. In the final installment of this year’s continuing education series (brought to you with generous support from Colgate-Palmolive), AAP members Ranjitha Krishna, MPH, MS, DMD, and Roger M. Arce, DDS, MS, PhD, provide insight into this important topic and how dental hygienists can contribute to the health of both pregnant women and their babies.
—Joan Otomo-Corgel, DDS, MPH,
President, American Academy of Periodontology
Clinical Professor, Department of Periodontics, University of California, Los Angeles, School of Dentistry
Diverse pregnancy outcomes usually include several different Disease outcomes following pregnancy, including low-birth Weight (≤5.5 lbs), very low birth weight (≤3.3 lbs), preterm birth/delivery (≤37 weeks of gestation) or very preterm (≤32 weeks of gestation) birth, preeclampsia (maternal hypertension/proteinuria), and miscarriage/ still births. While several of these outcomes can be interdependent, it is still unclear if they share common pathological processes or associated risk factors. One of the common risk factors for adverse pregnancy outcomes is maternal infection, which is believed to account for most of preterm delivery cases (25% to 40%).1
The primary etiology of virtually all periodontal diseases is bacterial biofilm, or dental plaque that forms on tooth surfaces. Periodontal infections are caused by anaerobic, Gram-negative bacteria, including Aggregatibacter actinomycetemcomitans, Tannerella forsythensis, Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, Campylobacter rectus, and Treponema denticola. Periodontal diseases are also caused by the complex interplay between the bacterial products and the host response that are modified by behavioral and/or systemic factors.2 About 50% of the tissue destruction associated with periodontal diseases is attributed to host response. The immune response that develops due to the chronic presence of plaque bacteria results in destruction of structural components of the periodontium—leading to the clinical signs of periodontitis and, ultimately, tooth loss.
![]() GLOBAL PUBLIC HEALTH BURDEN
GLOBAL PUBLIC HEALTH BURDEN
Worldwide, approximately 1.1 million neonates die every year from complications of preterm birth. Preterm birth is the second most common global cause of death in children younger than 5, after pneumonia.3 Preterm birth and low-birth weight are also the leading risk factors for deaths due to neonatal infections, and they contribute to long-term growth, cognitive, visual, and learning impairments.4 Recent epidemiological analyses estimate that the average cost of preterm birth in the United States is approximately $3 billion per year, or $35,471 per preterm birth—despite comprehensive health policy interventions.5 The prevalence of preterm birth seems to be increasing worldwide, but the reasons for this phenomenon are not clear. Maternal conditions, such as teenage pregnancy, chronic conditions (diabetes, obesity), and maternal clinical/subclinical infections (vaginal/oral), may partially explain the increased prevalence. Currently, there are not many preventive interventions available to overcome these maternal conditions, and it remains unknown whether interventions can reduce their occurrence.5
Preeclampsia is a multisystem disorder that occurs during pregnancy and the post-partum period. Affecting both the mother and the fetus, preeclampsia is defined as a first-time hypertension episode and proteinuria in a previously normotensive woman. Women with preeclampsia are at an increased risk for maternal and/or fetal morbidity and mortality.6 Preeclampsia occurs in up to 7.5% of pregnancies worldwide.7 In the US, the prevalence of preeclampsia is about 3.4%, and it is two times more likely to occur in first pregnancies.8 The associated risk factors for preeclampsia include past history of preeclampsia, first pregnancy, familial history, pregestational diabetes, obesity, chronic kidney disease, twin pregnancies, advanced maternal age, and maternal infections.7 Women with preeclampsia develop high blood pressure, swelling, sudden weight gain, headaches, and vision changes. Typically, preeclampsia occurs after 20 weeks of gestation and up
to 6 weeks after delivery.8 Women with preeclampsia are at an increased risk for life-threatening events, such as placental abruption, acute renal failure, cerebral hemorrhage, hepatic failure or rupture, pulmonary edema, disseminated intravascular coagulation, and progression to eclampsia (development of seizures after
severe preeclampsia). Globally, 10% to 15% of maternal deaths are attributed to preeclampsia.9 In the US, preeclampsia is one of the three leading causes of maternal death, along with hemorrhage and thromboembolism.10,11
Spontaneous abortion or miscarriage is defined as a clinically recognized loss of pregnancy before the 20th week of gestation, with the fetus typically weighing 1 lb or less.7,12 Miscarriage is the most common complication of early pregnancy, with a prevalence of 8% to 20% globally; however, the incidence widely varies with
maternal age and ethnicity.13 Numerous risk factors are associated with an increased risk of miscarriage, including older maternal age; previous miscarriage; smoking; alcohol and cocaine use; excessive intake of caffeine; and use of nonsteroidal anti-inflammatory drugs. Spontaneous abortion can also be caused by chromosomal
abnormalities in the embryo, exposure to teratogens, or severe infection/sepsis episodes.14
ROLE OF PERIODONTAL DISEASES
The association between adverse pregnancy outcomes and periodontal diseases is subject to scientific debate. Extensive research has been conducted to evaluate the associations between adverse pregnancy outcomes and periodontal diseases. Even though there is significant variability in study design, definitions, and control of confounding factors, it is clear that periodontal diseases are a modest, but independent risk factor for adverse pregnancy outcomes in several populations.15 Despite the positive epidemiological associations, there is contradictory evidence regarding whether ad verse pregnancy outcomes are reduced when periodontal diseases are
treated during pregnancy.16 In other words, nonsurgical periodontal therapy does not seem to reduce preterm birth incidence when compared to untreated pregnant women. The results from large multicenter randomized controlled clinical trials (RCTs) that included more than 1,300 pregnant women did not demonstrate better obstetric outcomes following periodontal therapy.17,18 Other RCTs have reported beneficial effects of nonsurgical periodontal therapy in reducing adverse pregnancy outcomes—specifically a reduction in preterm births.19,20
In general, women do not seem to respond satisfactorily to conventional periodontal therapy during pregnancy, as periodontal inflammation does not completely resolve until after delivery.21,22 Women are more susceptible to gingival and periodontal inflammation due to hormonal changes during both menstrual cycles and
pregnancy.23 If pathogenic features associated with adverse pregnancy outcomes are developed during the early stages of pregnancy, then conventional periodontal therapy after the first trimester will have limited effects.24 The real effect of periodontal disease treatment may lie in prevention of the disease before conception. 25 Addressing gingival and periodontal inflammation before conception and throughout pregnancy and delivery may shed further light on this issue. For example, recent trials evaluating intensive oral hygiene instructions during early pregnancy have reported a significant decrease in overall gingival inflammation and improved periodontal health.26 Also, a more preventive and personalized targeted therapy could have a more predictive outcome.27 Despite the negative results after large clinical trials, treatment of periodontal diseases during the second trimester is safe and continues to be recommended as part of prenatal care.28
PATHOGENIC MECHANISMS
Periodontal disease-associated bacteria have been linked to adverse pregnancy outcomes. Important periodontal pathogens, such as P. gingivalis, F. nucleatum, and C. rectus, have been detected in human placentas of women with preterm delivery,29 preeclampsia,30 and in the amniotic fluid of women with premature labor31 or premature labor with intact membranes. 32–34 In fact, the human placenta harbors a mix of bacteria (microbiome) similar to the micro biome in an adult human’s mouth.35 Fetal exposure to periodontal pathogens from maternal oral biofilms also has been demonstrated by measuring fetal immunoglobulin M, which specifically reacts to P. gingivalis and C. rectus, suggesting adaptive immune responses developed by the fetus
against oral bacteremia from the mother.27,36
During pregnancy, the placenta invades and grows through support of the maternal uterine tissue. Because of its high vascularity, the placenta is able to exchange nutrients from the maternal tissue and carry it to the fetus via the umbilical cord. The fetus is in the amniotic fluid, surrounded by the amniotic sac. As the fetus continues to grow, there is less space and nutrients available for its growth. As pregnancy progresses, the levels of prosta glandin E2 and inflammatory cytokines, such as TNF-alpha and IL-1 beta, rise steadily until they induce the rupture of the amniotic sac and cause uterine contraction, cervical dilation, and normal delivery.37 Thus, the pathway of inflammatory signaling controls normal childbirth.
Increased vascular permeability during pregnancy may facilitate the systemic circulation of the pathogens and their toxic byproducts to the feto-placental unit. This type of bacterial challenge induces the release of inflammatory cytokines, which can result in premature rupture of the membrane and early intrauterine contractions. Consequently, placental infection/inflammation is a key pathogenesis feature of most pregnancy complications. Laboratory evidence suggests that placental cells develop inflammation when exposed to F. nucleatum and C. rectus.38 Moreover, evidence derived from animal experiments has demonstrated bacterial translocation, placental inflammation, increased fetal resorption (similar to miscarriage), and/or growth restriction in response to infection with oral pathogens.39–41
A large number of studies associate an increase in the levels of local and systemic markers of inflammation—such as CRP, IL-1Beta, IL-6, TNF-alpha, PGE3, fibronectin, and alphafetoprotein—with adverse pregnancy outcomes. Besides preterm birth, elevated levels of CRP are also associated with intrauterine growth restriction42 and preeclampsia.43,44 Preeclampsia is associated with increases in maternal blood pressure, maternal proteinuria, and levels of pro-inflammatory cytokines.44 Increased levels of IL-6, CRP, and TNFalpha also interfere with insulin signaling and cause glucose intolerance that can result in gestational diabetes.45
Periodontal diseases are infectious diseases that occur at a distance from the feto-placental unit. As in any infection, there is an increase in the local inflammatory response against the bacteria and their virulence factors in the oral cavity. Eventually these cytokines, as well as the bacteria, can enter the blood circulation and disseminate throughout the body—triggering a systemic inflammatory response.39,46 A model explaining the possible association between periodontal disease and adverse pregnancy outcomes was recently proposed.27 According to this hypothetical model, periodontal diseases may take either a direct pathway, in which periodontal bacteria (and their virulence products) disseminate to the feto-placental unit where they initiate an ectopic infection and trigger a local inflammatory response that results in the elevation of inflammatory cytokines and mediators that contribute to pregnancy complications; or an indirect pathway, in which inflammatory cytokines and mediators produced at the gingival level enter the blood stream, leading to a systemic inflammatory response that reaches the feto-placental unit and triggers adverse pregnancy outcomes.
Abundant evidence from animal models and in vitro experiments support this hypothetical model. However, it remains unclear if the model applies to the association of periodontal disease and adverse pregnancy outcomes in humans (Figure 1).27
![]() IMPLICATIONS FOR DENTAL HYGIENE
IMPLICATIONS FOR DENTAL HYGIENE
Encouraging prevention and minimizing oral diseases before and during pregnancy are key to reducing adverse pregnancy outcomes. The pregnancy period is a time when women are highly motivated to adopt healthy behaviors, providing an opportunity to emphasize the importance of effective oral self-care.47 Furthermore, women of low socioeconomic status are more likely to obtain dental care because of Medicaid insurance assistance that accompanies prenatal medical/dental coverage. This is of particular importance when approximately 40% of pregnant women have some form of periodontal disease.48 According to the Committee on Health Care for Underserved Women from the American College of Obstetricians and Gynecologists, 35% of American women reported not having a dental visit within 1 year and 56% of women do not visit a dentist during pregnancy.47
Oral health is an important component of general health and should be maintained during a woman’s lifespan, especially during pregnancy. Despite the lack of evidence for a
direct causal relationship between periodontal disease and adverse pregnancy conditions, the treatment of periodontal diseases during pregnancy is not associated with adverse maternal and/or birth outcomes.17,18,49 Moreover, prenatal periodontal therapy is associated with the improvement of maternal oral health28 and the reduction of cariogenic bacterial transmission to children.50
Several physiologic changes during pregnancy can result in noticeable changes in the periodontium, such as pregnancy gingivitis, benign oral gingival lesions (eg, pyogenic granuloma),
tooth mobility, tooth erosion, dental caries, and periodontitis. 51,52 Dental hygienists can play a significant role in the early detection and treatment of these problems. Recent evidence also suggests that individually-tailored one-on-one oral hygiene counseling combined with an in-office oral hygiene regimen (dental prophylaxis) effectively reduce gingival inflammation in pregnant patients 8 weeks after intervention.53 The same approach also significantly reduces the local/systemic levels of pro-inflammatory cytokines, which suggests a beneficial systemic effect.26 As such, women need to be educated about the possibility of these changes occurring during pregnancy and the need for good oral health habits and professional treatment to maintain dental health.
![]() CONCLUSION
CONCLUSION
According to a report presented by the European Federation of Periodontology and the American Academy of Periodontology,54 the main variables that provide direct assessment of oral infectious exposure to the fetal/placental unit are the presence/quantity of microorganisms and microbial components in the amniotic fluid and placental cord blood, as well as the presence of inflammatory cytokines in maternal serum, fetal cord blood, and amniotic fluid. Oral pathogens, such as F. nucleatum, C. rectus, P. gingivalis, and Bergeyella spp., are strongly associated to adverse pregnancy outcomes. A 2011 systematic review found a positive association between periodontal diseases and adverse pregnancy outcomes in nine out of 11 studies.19 The authors concluded that there was not enough evidence to support the theory that maternal periodontal treatment can decrease the risk of preterm birth or low-birth weight, but they did recommend that pregnant women be instructed on the importance of maintaining their periodontal health and that obstetricians be encouraged to refer patients for periodontal treatment as part of routine prenatal evaluations. While periodontal treatment during pregnancy is safe and leads to better periodontal conditions, strong evidence shows that scaling and root planing does not reduce the overall rates of preterm birth and low-birth weight.49,54 Similarly, several systematic reviews and meta-analyses do not support the theory that periodontal treatment reduces the risk of preterm birth.50,55–57 Conversely, two meta-analyses found a significant reduction in the risk of preterm birth following periodontal treatment in a subgroup of high-risk women.58–60 The latter suggests that women at elevated risk of adverse pregnancy outcomes may benefit from periodontal treatment, and that they should be screened for periodontal diseases.
The biological models that explain the association between periodontal diseases and adverse pregnancy outcomes are mostly derived from animal studies and in-vitro experiments and may not be completely applicable to humans. Also, some human studies have shown that not all pregnant women with similar microbiological profiles present comparable fetal exposure to the periodontal pathogens. Future therapy should be designed based on virulence potential of different pathogens, as well as the detection of a susceptible host immune response. Therefore, a more preventive and personalized approach to treating pregnant women could have a more predictive outcome when compared to the traditional “one-treatment-fits-all” periodontal therapy.27,61
In spite of the controversial results, prevention remains the ultimate goal in the association between periodontal diseases and adverse pregnancy outcomes. Consequently, the dental hygiene team has a great opportunity to directly impact the oral and systemic health of pregnant women.
REFERENCES
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, HassanS. The role of inflammation and infection in preterm birth. SeminReprod Med. 2007;25:21–39.
- Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruptionof microbial homeostasis. Unlearning learned concepts. Periodontol2000. 2013;62:203–217.
- Liu L, Johnson HL, Cousens S, et al. Global, regional, and nationalcauses of child mortality: an updated systematic analysis for 2010with time trends since 2000. Lancet. 2012;379:2151–2161.
- Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering T.4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900.
- Chang HH, Larson J, Blencowe H, et al. Preventing preterm births:analysis of trends and potential reductions with interventions in 39countries with very high human development index. Lancet.2013;381:223–234.
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of preeclampsiaand the other hypertensive disorders of pregnancy. BestPract Res Clin Obstet Gynaecol. 2011;25:391–403.
- Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the ratesof preeclampsia, eclampsia, and gestational hypertension, UnitedStates, 1987-2004. Am J Hypertens. 2008;21:521–526.
- Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in theUnited States, 1980-2010: age-period-cohort analysis. BMJ.2013;347:6564.
- Duley L. The global impact of pre-eclampsia and eclampsia. SeminPerinatol. 2009;33:130–137.
- Main EK. Maternal mortality: new strategies for measurementand prevention. Curr Opin Obstet Gynecol. 2010;22:511–516.
- Mac KAP, Berg CJ, Liu X, Duran C, Hoyert DL. Changes in pregnancymortality ascertainment: United States, 1999-2005. ObstetGynecol. 2011;118(1):104-10.
- Regan L, Rai R. Epidemiology and the medical causes of miscarriage.Baillieres Best Pract Res Clin Obstet Gynaecol.2000;14:839–854.
- Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception,early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577–584.
- Morgan J, Roberts S. Maternal sepsis. Obstet Gynecol Clin NorthAm. 2013;40:69–87.
- Ide M, Papapanou PN. Epidemiology of association betweenmaternal periodontal disease and adverse pregnancy outcomes—systematic review. J Periodontol. 2013;84(Suppl 4):S181–S194.
- Wimmer G, Pihlstrom BL. A critical assessment of adverse pregnancyoutcome and periodontal disease. J Clin Periodontol.2008;35(Suppl 8):380–397.
- Offenbacher S, Beck JD, Jared HL, et al. Effects of periodontaltherapy on rate of preterm delivery: a randomized controlled trial.Obstet Gynecol. 2009;114:551–559.
- Michalowicz BS, Hodges JS, DiAngelis AJ, et al. Treatment ofperiodontal disease and the risk of preterm birth. N Engl J Med.2006;355:1885–1894.
- Chambrone L, Pannuti CM, Guglielmetti MR, Chambrone LA. Evidencegrade associating periodontitis with preterm birth and/or lowbirth weight: II: a systematic review of randomized trials evaluatingthe effects of periodontal treatment. J Clin Periodontol.2011;38:902–914.
- Gazolla CM, Ribeiro A, Moyses MR, Oliveira LA, Pereira LJ, SallumAW. Evaluation of the incidence of preterm low birth weight inpatients undergoing periodontal therapy. J Periodontol.2007;78:842–848.
- Lopez NJ. Letter to the editor: re: intrapregnancy non-surgicalperiodontal treatment and pregnancy outcome: a randomized controlledtrial. J Periodontol. 2014;85:880–881.
- Pirie M, Irwin C, Linden GJ. Letter to the editor: authors’response. J Periodontol. 2014;85:881–883.
- Khosravisamani M, Maliji G, Seyfi S, et al. Effect of the menstrualcycle on inflammatory cytokines in the periodontium. J PeriodontalRes. 2014;49:770–776.
- Offenbacher S, Beck J. Has periodontal treatment failed to reduceadverse pregnancy outcomes? The answer may be premature. J Periodontol.2007;78:195–197.
- Jiang H, Xiong X, Su Y, et al. A randomized controlled trial ofpre-conception treatment for periodontal disease to improve periodontalstatus during pregnancy and birth outcomes. BMC PregnancyChildbirth. 2013;13:228.
- Kaur M, Geisinger ML, Geurs NC, et al. Effect of intensive oralhygiene regimen during pregnancy on periodontal health, cytokinelevels, and pregnancy outcomes. A pilot study. J Periodontol.2014;31:1–14.
- Madianos PN, Bobetsis YA, Offenbacher S. Adverse pregnancyoutcomes (APOs) and periodontal disease: pathogenic mechanisms.J Periodontol. 2013;84(Suppl 4):S170–S180.
- Newnham JP, Newnham IA, Ball CM, et al. Treatment of periodontaldisease during pregnancy: a randomized controlled trial.Obstet Gynecol. 2009;114:1239–1248.
- Katz J, Chegini N, Shiverick KT, Lamont RJ. Localization of P. gingivalisin preterm delivery placenta. J Dent Res. 2009;88:575–578.
- Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidenceof periopathogenic microorganisms in placentas of womenwith preeclampsia. J Periodontol. 2007;78:670–676.
- Leon R, Silva N, Ovalle A, et al. Detection of Porphyromonas gingivalisin the amniotic fluid in pregnant women with a diagnosis ofthreatened premature labor. J Periodontol. 2007;78:1249–1255.
- Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D.Identification and sequencing of bacterial rDNAs in culture-negativeamniotic fluid from women in premature labor. Am J Perinatol.2004;21:319–323.
- Bohrer JC, Kamemoto LE, Almeida PG, Ogasawara KK. Acutechorioamnionitis at term caused by the oral pathogen Fusobacteriumnucleatum. Hawaii J Med Public Health. 2012;71:280–281.
- Han YW. Oral health and adverse pregnancy outcomes—what’snext? J Dent Res. 2011;90:289–293.
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J.The placenta harbors a unique microbiome. Sci Transl Med.2014;6:237–265.
- Madianos PN, Lieff S, Murtha AP, et al. Maternal periodontitisand prematurity. Part II: maternal infection and fetal exposure. AnnPeriodontol. 2001;6:175–182.
- Haram K, Mortensen JH, Wollen AL. Preterm delivery: anoverview. Acta Obstet Gynecol Scand. 2003;82:687–704.
- Arce RM, Diaz PI, Barros SP, et al. Characterization of the invasiveand inflammatory traits of oral Campylobacter rectus in amurine model of fetoplacental growth restriction and in trophoblastcultures. J Reprod Immunol. 2010;84:145–153.
- Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS.Fusobacterium nucleatum induces premature and term stillbirths inpregnant mice: implication of oral bacteria in preterm birth. InfectImmun. 2004;72:2272–2279.
- Offenbacher S, Riche EL, Barros SP, Bobetsis YA, Lin D, Beck JD.Effects of maternal Campylobacter rectus infection on murine placenta,fetal and neonatal survival, and brain development. J Periodontol.2005;76(Suppl 11):2133–2143.
- Yeo A, Smith MA, Lin D, et al. Campylobacter rectus mediatesgrowth restriction in pregnant mice. J Periodontol. 2005;76:551–557.
- Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB,van Wijk IJ. Elevated C-reactive protein levels during first trimester ofpregnancy are indicative of preeclampsia and intrauterine growthrestriction. J Reprod Immunol. 2003;59:29–37.
- Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P. Elevated C-reactive protein and pro-inflammatorycytokines in Andean women with pre-eclampsia. Int J GynaecolObstet. 2001;75:243–249.
- Herrera JA, Parra B, Herrera E, et al. Periodontal disease severityis related to high levels of C-reactive protein in pre-eclampsia. JHypertens. 2007;25:1459–1464.
- Bullon P, Jaramillo R, Santos-Garcia R, et al. Relation of periodontitisand metabolic syndrome with gestational glucose metabolismdisorder. J Periodontol. 2014;85:1–8.
- Han YW, Ikegami A, Bissada NF, Herbst M, Redline RW, AshmeadGG. Transmission of an uncultivated Bergeyella strain from theoral cavity to amniotic fluid in a case of preterm birth. J Clin Microbiol.2006;44:1475–1483.
- American College of Obstetricians and Gynecologists Women’sHealth Care Physicians; Committee on Health Care for UnderservedWomen. Committee Opinion No. 569: oral health care during pregnancyand through the lifespan. Obstet Gynecol. 2013;122:417–422.
- Lieff S, Boggess KA, Murtha AP, et al. The oral conditions andpregnancy study: periodontal status of a cohort of pregnant women.J Periodontol. 2004;75:116–126.
- Michalowicz BS, Gustafsson A, Thumbigere-Math V, Buhlin K.The effects of periodontal treatment on pregnancy outcomes. J Periodontol.2013;84(Suppl 4):S195–S208.
- Leader D. Periodontal disease treatment does not affect pregnancyoutcomes. J Am Dent Assoc. 2014;145:757–759.
- Silk H, Douglass AB, Douglass JM, Silk L. Oral health during pregnancy.Am Fam Physician. 2008;77:1139–1144.
- Boggess KA, Society for Maternal-Fetal Medicine PublicationsCommittee. Maternal oral health in pregnancy. Obstet Gynecol.2008;111:976–986.
- Geisinger ML, Geurs NC, Bain JL, et al. Oral health education andtherapy reduces gingivitis during pregnancy. J Clin Periodontol.2014;41:141–148.
- Sanz M, Kornman K. Periodontitis and adverse pregnancy outcomes:consensus report of the Joint EFP/AAP Workshop on Periodontitisand Systemic Diseases. J Periodontol. 2013;84(Suppl 4):S164–S169.
- Fogacci MF, Vettore MV, Leao AT. The effect of periodontal therapyon preterm low birth weight: a meta-analysis. Obstet Gynecol.2011;117:153–165.
- Khader YS, Ta’ani Q. Periodontal diseases and the risk of pretermbirth and low birth weight: a meta-analysis. J Periodontol.2005;76:161–165.
- Polyzos NP, Polyzos IP, Zavos A, et al. Obstetric outcomes aftertreatment of periodontal disease during pregnancy: systematicreview and meta-analysis. BMJ. 2010;341:7017.
- George A, Shamim S, Johnson M, et al. Periodontal treatmentduring pregnancy and birth outcomes: a meta-analysis of randomisedtrials. Int J Evid Based Healthc. 2011;9:122–147.
- Kim AJ, Lo AJ, Pullin DA, Thornton-Johnson DS, Karimbux NY.Scaling and root planing treatment for periodontitis to reducepreterm birth and low birth weight: a systematic review and metaanalysisof randomized controlled trials. J Periodontol.2012;83:1508–1519.
- Dasanayake AP. Scaling and root planing is effective in reducingpreterm birth only in high-risk groups. J Evid Based Dent Pract.2013;13:42–44.
- Kornman KS, Duff GW. Personalized medicine: will dentistry ridethe wave or watch from the beach? J Dent Res. 2012;91(Suppl 7):8S–11S.
From Dimensions of Dental Hygiene. October 2014;12(10):59–64.


 GLOBAL PUBLIC HEALTH BURDEN
GLOBAL PUBLIC HEALTH BURDEN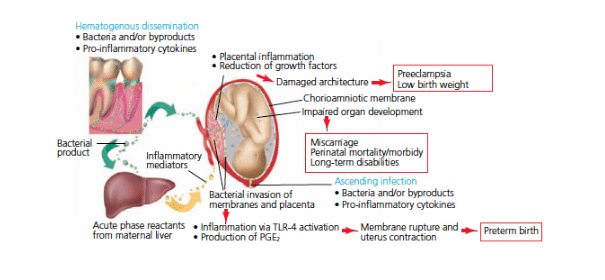 IMPLICATIONS FOR DENTAL HYGIENE
IMPLICATIONS FOR DENTAL HYGIENE CONCLUSION
CONCLUSION
