Advances in the Science of Fluoride
Newly formulated stannous fluoride stabilized with nitrate and phosphates offers clinicians a valuable tool in the fight against plaque biofilm.
This course was published in the March/April 2025 issue and expires April 2028. The author discloses an honorarium from Colgate-Palmolive Co. This 2 credit hour self-study activity is electronically mediated, and is supported through an unrestricted educational grant from Colgate.
AGD Subject Code: 490
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the role of oral biofilm in the development of dental caries, gingivitis, and periodontal diseases.
- Discuss the effectiveness of stannous fluoride as an antimicrobial agent in dentifrices.
- List the challenges associated with stannous fluoride stabilization in dentifrice formulations and discuss recent advancements in improving its bioavailability, efficacy, and patient compliance.
Introduction
As the prevention specialists, we know that effective biofilm management is fundamental to preventing dental caries and periodontal diseases. Unfortunately, how many times have we been frustrated by our patients’ lack of compliance? That’s why selecting the most effective oral hygiene tools tailored to patients’ individual needs is one of the best ways we can support their long-term oral health.
Stannous fluoride (SnF₂) stands out due to its dual-action benefits: enamel protection and antibacterial properties. The challenge? Historically, keeping SnF₂ stable in toothpaste formulations has been tricky, which can limit its effectiveness.
Thankfully, advancements in formulation technology have led to a next-generation stannous fluoride toothpaste that ensures high bioavailability and extends its therapeutic benefits. By incorporating stabilizing agents, this innovative formula maintains the potency of SnF₂, delivering sustained antibacterial activity.
When patients use a stabilized SnF₂ toothpaste as part of their daily routine, they can experience better biofilm control and reduced inflammation. And as the go-to experts in prevention, dental hygienists are in the perfect position to help patients choose evidence-based solutions that make a real difference in their oral health.
— Phyllis Martina, RDH, MBA Senior Professional Education Manager Colgate Oral Pharmaceuticals, Inc.
Strategies for Mitigating Oral Biofilm
The effective removal of oral biofilm from tooth surfaces and surrounding soft tissues is essential for preventing and managing dental diseases.1 Maintaining oral health through the reduction of biofilm extends beyond routine toothbrushing and flossing. A healthy oral microbiome relies on daily efforts to minimize the proliferation of opportunistic pathogenic bacteria that can disrupt microbial balance and the breakdown of hard and soft oral tissues.
While oral hygiene measures are critical for disease prevention, they are often insufficient due to suboptimal self-care practices. Although patients should brush for 2 minutes twice daily, research indicates that the average brushing duration is only 45 seconds.2
Given these challenges, the mechanical disruption of biofilm is not sufficient to manage pathogenic bacterial growth. Over-the-counter dentifrices serve as an adjunct to mechanical biofilm removal — however, the efficacy of these formulations warrants further examination. Fluoride’s efficacy in the oral environment is influenced by multiple factors, including concentration, formulation, and salivary secretion rate. Furthermore, the retention of fluoride in plaque may be affected by the chemical composition of the fluoride source.3
Oral biofilm is a structured colony of bacteria residing in an extracellular matrix produced by the bacteria. The adhesive nature of the extracellular matrix allows it to firmly attach to oral hard and soft tissues.4 These communities consist of bacteria, polysaccharides, proteins, and extracellular DNA. Plaque serves as a protective environment that fosters bacterial persistence.4 Differences in microbial composition and virulence depend on the surface properties to which the biofilm binds and the localized environmental conditions of each site.5
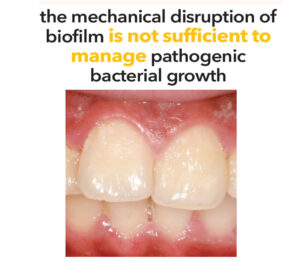 The initial adhesion phase of oral biofilm formation begins immediately after oral hygiene practices are completed. Early aerobic colonizers, such as Streptococcus, bind to the dental pellicle on tooth surfaces.4 As the biofilm matures, anaerobic bacteria, such as Porphyromonas gingivalis, may proliferate under certain conditions, which increases their virulence and triggers an inflammatory cascade. As these microbial networks develop through the congregation and replication of various bacterial species, they establish a structured living system that continues to thrive and eventually disperses to form additional colonies throughout the oral cavity.6
The initial adhesion phase of oral biofilm formation begins immediately after oral hygiene practices are completed. Early aerobic colonizers, such as Streptococcus, bind to the dental pellicle on tooth surfaces.4 As the biofilm matures, anaerobic bacteria, such as Porphyromonas gingivalis, may proliferate under certain conditions, which increases their virulence and triggers an inflammatory cascade. As these microbial networks develop through the congregation and replication of various bacterial species, they establish a structured living system that continues to thrive and eventually disperses to form additional colonies throughout the oral cavity.6
These bacterial communities evolve rapidly in the presence of a continuous fluid flow and a nutrient-rich setting. Saliva provides essential proteins, electrolytes, and enzymes, while dietary carbohydrates serve as a primary energy source.7 The intricate biofilm matrix offers colonizing species advantages, including protection from host immune defenses and antimicrobial agents, while facilitating co-aggregation between complementary bacterial species and microbial interactions.4
The oral microbiome comprises more than 700 species, including acidogenic, aciduric, Gram-positive, and Gram-negative bacteria, that contribute to the commensal oral environment.8 Acidogenic and aciduric bacteria metabolize carbohydrates, producing acid as a byproduct that lowers pH levels in the oral cavity. Their ability to survive and thrive in an acidic environment accelerates the development of dental caries and periodontal diseases. P. gingivalis, on the other hand, plays a significant role in gum disease by initiating the breakdown of the periodontium and contributing to the onset and progression of gingivitis, which if left untreated, may lead to the more severe condition: periodontal disease.7-9
Oral biofilms also incorporate yeasts, protozoa, Archaea, and viruses.9,10 The commensal interactions between bacteria are essential for the preservation of symbiotic balance in the oral cavity. As a result of fluctuations in nutrient availability, acidic pH, and oxidative stress, microbial dysbiosis develops, resulting in the start and progression of oral diseases.9
The fluoride used in many dentifrices include sodium fluoride (NaF), sodium monofluorophosphate (MFP), and stannous fluoride (SnF₂), all of which promote remineralization and reduce the risk of caries by enhancing the tooth’s resistance to acid attacks.11 In addition to providing hard tissue benefits, SnF₂ offers antibacterial protection by altering the cell walls of pathogenic bacteria associated with caries, gingivitis, periodontal diseases, and oral malodor.12-14 The antibacterial action enhances overall gingival health by reducing bacterial virulence and the associated inflammatory responses.12,15
How Stannous Fluoride Works
SnF₂ is a tin-based fluoride compound, which in dentifrice is typically formulated at a concentration of 0.454%. SnF₂’s effectiveness in preventing dental caries is derived largely from fluoride and its antibacterial efficacy is derived from the stannous ions (Sn+₂). Exposure of Sn+₂ to air, water, or heat can lead to the oxidation of stannous ions to stannic ions (Sn+4), which are inactive against bacteria. Thus it is critical to properly formulate a SnF₂ dentifrice to maintain its antibacterial efficacy.16
SnF₂ is water-soluble and hydrolyzes into fluoride ions and tin hydroxide. These chemical changes affect the bioavailability of SnF₂, leading to deactivation and a loss of therapeutic effects.16 The retention of stannous ions is key to its effectiveness, as these ions disrupt bacterial metabolism, inhibit bacterial growth, reduce cellular respiration, and prevent bacterial acid production during glycolysis.17
Included in the United States Food and Drug Administration over-the-counter drug monograph for its evidence in anticaries and antigingivitis effectiveness,18 SnF₂’s antibacterial efficacy has been extensively studied, demonstrating safety and efficacy. To overcome stability challenges, researchers have explored methods such as removing water, incorporating stannous chloride to maintain a stannous reservoir, and adding chelating agents such as polyphosphates and citrates.19,20
While SnF₂ dentifrices have the potential to cause extrinsic staining due to oxidation from improper stabilization, modern formulations have significantly minimized this effect.20 To prevent oxidation, manufacturers reduce or eliminate water, as SnF₂ is unstable in high-water concentrations. However, lowering the water content can create a messy, stringy texture and an unpleasant taste, which may reduce patient compliance.19,20
The use of stabilizers can help preserve stannous ions for optimal uptake; however, this may also affect the texture and taste of the dentifrice. Another approach involves adding stannous salts, such as stannous chloride, to replenish lost stannous ions, but this has proven ineffective as a high proportion of inactive stannic ions still forms.21,22 Recently, dentifrice manufacturers have created more sophisticated formulations that effectively stabilize SnF₂.
Research Results
The advancement of imaging techniques and microbiome analysis has enabled researchers to examine the biological changes in oral biofilms subjected to SnF₂, providing a more precise comparison of different fluoride compositions in dentifrices. Research findings indicate that SnF₂-containing toothpaste modifies the biofilm architecture and gene expression, resulting in a less adhesive and less virulent biofilm.4,12
Chakraborty et al17 performed an in vitro study and randomized controlled trial to assess the effectiveness of 0.454% SnF₂ toothpaste stabilized with nitrate and phosphates (SNaP). The in vitro testing assessed the prolonged antimicrobial efficacy of the SNaP toothpaste on cultured salivary biofilms, juxtaposing it with other prevalent over-the-counter toothpastes: one comprising 0.454% SnF₂ containing sodium gluconate (SnF₂ + SG) and another containing 0.24% NaF and 5% potassium nitrate.
 The study investigated how different types of dentifrices affect bacterial respiration and glycolysis, key processes for bacterial energy production. The terms oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were used to assess how bacteria metabolize oxygen and produce acid — key indicators of bacterial activity.
The study investigated how different types of dentifrices affect bacterial respiration and glycolysis, key processes for bacterial energy production. The terms oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were used to assess how bacteria metabolize oxygen and produce acid — key indicators of bacterial activity.
Antibacterial performance was measured by monitoring bacterial metabolic function, specifically bacterial respiration via OCR and glycolysis via ECAR over a 200-minute observation period. The SNaP results showed sustained OCR suppression for the entire 200 minutes, while SnF₂ + SG demonstrated suppression for up to 50 minutes. SNaP also maintained near-zero levels of ECAR throughout the observation period. Both SNaP and SnF₂ + SG outperformed NaF in both assessments, but SNaP exhibited more significant superiority over SnF₂ + SG in both measures.
The randomized controlled study by Chakraborty et al17 employed a double-blind, single-center, two-arm, parallel design to assess the effectiveness of SNaP and a dentifrice containing 0.76% MFP in 100 healthy participants over 5 weeks. Participants were instructed to brush twice daily for 2 minutes using a full ribbon of toothpaste. Additionally, they were advised to avoid any supplementary oral hygiene products throughout the study period. Oral samples of saliva, supragingival plaque, tongue scrapings, and buccal mucosa and gingival scrapings were collected for examination.
Findings revealed a significant difference between the SNaP and MFP groups. The SNaP group exhibited markedly lower bacterial loads in plaque, tongue, cheek, gingiva, and saliva samples 12 hours post-brushing during the second and fourth weeks of sample collection. Additionally, use of the SNaP dentifrice demonstrated extended bioavailability of SnF₂ and its antibacterial components, which supports prolonged therapeutic effects and prevents soft tissue niches from harboring microbial reservoirs that typically redeposit bacteria on teeth after cleansing.17
Another study by Lee et al19 investigated the clinical efficacy of SNaP on dental plaque and gingivitis using a randomized controlled trial over 6 months. In the end, 77 participants completed the study. The following indices were used to assess and compare samples at 3- and 6-month intervals: gingival index, gingival severity, gingival interproximal, plaque index, plaque severity, and plaque interproximal.
Participants’ indices were reassessed at the 3-month interval, and the SNaP dentifrice demonstrated significant superiority over 0.76% MFP. While MFP showed a reduction from baseline in all plaque indices, it did not reduce gingival indices. In contrast, those who used the SNaP dentifrice exhibited reductions in both plaque and gingival indices.
At the 6-month interval, MFP showed reductions from baseline in all plaque and gingival indices except for the gingival interproximal index. Meanwhile, the SNaP dentifrice continued to demonstrate reductions across all plaque and gingival indices, including the gingival interproximal index.19
Dental Caries and Periodontal Pathogens
Dental caries is a biofilm-dependent oral disease caused by cariogenic pathogens, such as Streptococcus mutans, and is associated with frequent exposure to a sugar-rich diet and inadequate oral hygiene. S. mutans plays a significant role in forming complex extracellular matrices, contributing to an increasingly acidogenic bacterial environment within the biofilm as caries progress.23 Understanding the pathogenesis of caries is essential for developing dentifrices that can effectively target bacteria.
SnF₂ dentifrice is beneficial in managing gingivitis primarily through its antimicrobial effects on oral bacterial flora. It reduces biofilm accumulation and moderates metabolic activity.13 Additionally, SnF₂ enhances its antibacterial action by binding directly to endotoxins, such as lipopolysaccharide (LPS), thereby suppressing the activation of Toll-like receptors — key initiators of signaling pathways that contribute to gingival inflammation.24,25
- gingivalis possesses LPS on its outer membrane — a highly toxic component that triggers the host immune response. LPS is critical for maintaining bacterial cell integrity and function while serving as a protective barrier against host defenses.24,25 The outer membrane contains endocytic-derived vesicles that facilitate toxin transport and enhance pathogenicity. P. gingivalis utilizes outer membrane vesicles to communicate, manipulate the immune system, and disrupt normal host responses, further driving periodontal disease progression.24,25 Therefore, SnF₂ dentifrice plays a crucial role in mitigating these effects and supporting oral health.
Dentinal Hypersensitivity
Dentinal hypersensitivity — a brief, acute pain that emanates from the exposed dentin in response to thermal, tactile, osmotic, chemical, or evaporative stimuli — poses a challenge for oral health professionals.26 Liu et al27 compared the effectiveness of dentin occlusion and pain relief among a SNaP dentifrice, a 0.24% NaF + 5% potassium nitrate desensitizing dentifrice, and a nondesensitizing 0.76% MFP dentifrice. Clinical trial results demonstrated that SNaP was more effective at reducing tactile hypersensitivity. The in vitro results showed 86% occlusion for SNaP, compared to 35% occlusion for 0.76% MFP.
SNaP toothpaste significantly outperformed both the positive and negative control groups in reducing tactile hypersensitivity, demonstrating rapid and sustained effectiveness in managing dentin hypersensitivity. The improvements were statistically significant, confirming the highly effective test formulation.27 The SNaP dentifrice also demonstrated effectiveness in reducing air blast hypersensitivity compared to other commercially available dentifrices.
Conclusion
SnF₂ has proven to be a valuable tool in modern oral care, offering multifaceted benefits that extend beyond caries prevention. Its ability to provide antibacterial protection, reduce gingival inflammation, strengthen enamel, and alleviate dentinal hypersensitivity makes it a key ingredient in effective dentifrices. While proper stabilization is necessary to maintain its therapeutic potential, advancements in formulation continue to enhance its efficacy and patient acceptance.
References
- Arweiler NB, Netuschil L. The oral microbiota. Adv Exp Med Biol. 2016:902:45-60.
- Creeth JE, Gallagher A, Sowinski J, et al. The effect of brushing time and dentifrice on dental plaque removal in vivo. J Dent Hyg. 2009;83:111-116.
- Geisinger ML, Geurs NC, Novy B, et al. A randomized double-blind clinical trial evaluating comparative plaque and gingival health associated with commercially available stannous fluoride-containing dentifrices as compared to a sodium fluoride control dentifriceJ J Periodontol. 2023;94:1112-1121.
- Bertolini M, Costa R, Barão V, et al. Oral microorganisms and biofilms: new insights to defeat the main etiologic factor of oral diseases. Microorganisms. 2022;10:2413.
- Xu X, He J, Xue J, et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol. 2014;17:699-710.
- Rath S, Bal SCB, Dubey D. Oral biofilm: development mechanism, multidrug resistance, and their effective management with novel techniques. Rambam Maimonides Med J. 2021;12:e0004.
- Saini R, Saini S, Sharma S. Biofilm: A dental microbial infection. J Nat Sci Biol Med. 2011;2:71.
- Larsen T, Fiehn N. Dental biofilm infections — an update. Apmis. 2017;125:376-384.
- Spatafora G, Li Y, He X, Cowan A, Tanner ACR. The evolving microbiome of dental caries. Microorganisms. 2024;12:121.
- Mosaddad SA, Tahmasebi E, Yazdanian A, et al. Oral microbial biofilms: an update. Eur J Clin Microbiol Infect Dis. 2019;38:2005-2019.
- Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887-889.
- Gumber HK, Louyakis AS, Sarma T, et al. Effects of a stannous fluoride dentifrice on biofilm composition, gene expression and biomechanical properties. Microorganisms. 2022;10:1691.
- Xie S, Iberi V, Boissy Y, et al. Stannous fluoride forms aggregates between outer and inner membranes leading to membrane rupture of Porphyromonas gingivalis and Prevotella pallens. Front Oral Health. 2024;5:1427008.
- Johannsen A, Emilson CG, Johannsen G, Konradsson K, Lingström P, Ramberg P. Effects of stabilized stannous fluoride dentifrice on dental calculus, dental plaque, gingivitis, halitosis and stain: A systematic review. Heliyon. 2019;5:e02850.
- Fine N, Barbour A, Kaura K, et al. Effects of a stabilized stannous fluoride dentifrice on clinical, immunomodulatory, and microbial outcomes in a human experimental gingivitis model. J Periodontol. 2024;95:421-431.
- Myers CP, Pappas I, Makwana E, et al. Solving the problem with stannous fluoride: Formulation, stabilization, and antimicrobial action. J Am Dent Assoc. 2019;150:S5-S13.
- Chakraborty B, Seriwatanachai D, Triratana T, et al. Antibacterial effects of a novel stannous fluoride toothpaste stabilized with nitrate and phosphates (snap): in vitro study and randomized controlled trial. Compend Contin Educ Dent. 2024;45(Suppl 3):12-19.
- Pub Chem. Stannous fluoride. Available at http/://pubchem.ncbi.nlm.nih.gov/compound/Stannous-Fluoride. Accessed February 4, 2025.
- Lee S, Li Y, Mateo LR, et al. A 6-month randomized controlled trial to measure the efficacy of a stannous fluoride toothpaste stabilized with nitrate and phosphates (snap) on dental plaque and gingivitis. Compend Contin Educ Dent. 2024;45(Suppl 3):21-29.
- Manus LM, Myers CP, D’Ambrogio R, et al. The evolution of Colgate Total®: a new era stabilized by nitrate and phosphates. Compend Contin Educ Dent. 2024;45(Suppl 3):6-10.
- Ferretti GA, Tanzer JM, Tinanoff N. The effect of fluoride and stannous ions on Streptococcus mutans. Viability, growth, acid, glucan production, and adherence. Caries Res. 1982;16:298–307.
- Tinanoff N, Camosci DA. Microbiological, ultrastructural and spectroscopic analyses of the anti-tooth-plaque properties of fluoride compounds in vitro. Arch Oral Biol. 1980;25:531–543.
- Cai JN, Kim D. Biofilm ecology associated with dental caries: understanding of microbial interactions in oral communities leads to development of therapeutic strategies targeting cariogenic biofilms. Adv Appl Microbiol. 2023:122:27-75.
- Zhang Z, Liu D, Liu S, Zhang S, Pan Y. The role of Porphyromonas gingivalis outer membrane vesicles in periodontal disease and related systemic diseases. Front Cell Infect Microbiol. 2021;10:585917.
- Silva IL, Cascales E. Molecular strategies underlying Porphyromonas gingivalis virulence. J Mol Biol. 2021;433:166836.
- Zeola LF, Soares PV, Cunha-Cruz J. Prevalence of dentin hypersensitivity: systematic review and meta-analysis. J Dent. 2019;81:1-6.
- Liu Y, Lavender S, Ayad F, et al. Effect of a stannous fluoride toothpaste stabilized with nitrate and phosphates (SNAP) on dentin hypersensitivity: in vitro study and randomized controlled trial. Compend Contin Educ Dent. 2024;45(Suppl 3):30-39.
From Dimensions of Dental Hygiene. March/April 2025; 23(2):27-31.



