 SHIRONOSOV / ISTOCK / THINKSTOCK
SHIRONOSOV / ISTOCK / THINKSTOCK
Localized Aggressive Periodontitis
Early diagnosis, effective treatment, and patient compliance are integral to successfully managing this rapidly advancing disease.
This course was published in the February 2016 issue and expires February 28, 2019. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain the underlying pathogenesis of localized aggressive periodontitis (LAP).
- Identify the clinical features and appropriate diagnostic criteria of LAP.
- Discuss current treatment modalities and preventive measures for LAP.
INTRODUCTION
Aggressive periodontitis is much less common than chronic periodontitis and generally affects younger patients than the chronic form. The importance of early diagnosis among patients with localized aggressive periodontitis (LAP) cannot be overemphasized, as delays in detection may result in poor outcomes. This article provides a summary of this relatively rare form of periodontal disease that, despite clinicians’ best efforts, may lead to a decline in periodontal health and tooth loss. Soo-Woo Kim, DMD, DMSc, MS, provides an overview of LAP’s pathophysiology, prevalence, treatment, diagnosis, risk factors, and prevention strategies that may help clinicians identify and control modifiable risk factors. Although the amount of biofilm and calculus does not correlate with the level of disease in LAP, effective self-care, periodic dental hygiene visits, and oral hygiene instructions are essential parts of treatment. One of the most important recommendations for patients with LAP is to incorporate antimicrobial toothpaste into their oral hygiene regimens. The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support the article “Localized Aggressive Periodontitis” in collaboration with the American Academy of Periodontology.
—Matilde Hernandez, DDS, MS, MBA
Dental Science Liaison
Colgate Oral Pharmaceuticals
FROM THE AMERICAN ACADEMY OF PERIODONTOLOGY
Although the centers for disease control and prevention has found that the incidence of periodontal disease increases with age (70.1% of adults age 65 and older are said to have the disease), aggressive forms of periodontitis can affect children and young adults who are otherwise healthy. This condition, known as localized aggressive periodontitis, results in bone and attachment loss around the incisors and first molars. While rare, its presentation can have severe implications for those affected. In this article, “Localized Aggressive Periodontitis,” educator and Boston periodontist Soo-Woo Kim, DMD, DMSc, MS, highlights the importance of the condition’s early detection, risk factors, and treatment. The piece also highlights the value of taking preventive measures by individuals of all age groups. Central to periodontal disease prevention is the dental hygienist, whose guidance and care have helped patients all over
the world maintain the health of their teeth and gums. As always, the American Academy of Periodontology is proud to work with Dimensions of Dental Hygiene and Colgate-Palmolive to provide information that you can apply to your daily work with patients.
— Wayne Aldredge, DMD
President, American Academy of Periodontology
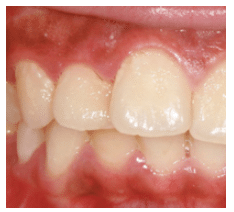
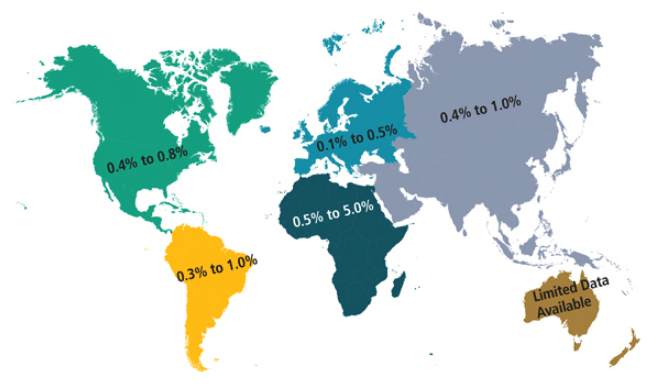
Localized aggressive periodontitis (LAP) Was First Described As “periodontosis”1 before it was labeled “localized juvenile periodontitis.” Due to the vague definition of “juvenile,” as well as the fact that the disease affects both children and adults, the American Academy of Periodontology (AAP) renamed the disease “localized aggressive periodontitis” in 1999.2 LAP is a form of inflammatory periodontal disease that typically presents with early loss of both periodontal attachment and bone proximate to the permanent incisors and first molars. The prevalence of LAP among those age 11 to 25 ranges from 0.4% to 0.8% in North America, 0.3% to 1.0% in South America, 0.1% to 0.5% in Western Europe, 0.5% to 5.0% in Africa, and 0.4% to 1.0% in Asia (Figure 1).3 Nonaggressive forms of periodontitis are 10 times more prevalent than aggressive periodontitis. LAP may affect both the primary and permanent dentition and runs in families.4 The clinical hallmarks of LAP comprise clinical attachment losses and vertical bony defects in incisor and first molar areas, a relative lack of calculus, and understated signs of clinical inflammation.
PATHOPHYSIOLOGY
The pathogenesis of LAP and its causative factors remain unclear.5,6 Besides its aggressive nature, the development of LAP is similar to that of chronic periodontitis. LAP begins with bacterial infection that may provoke an exaggerated host immune response.
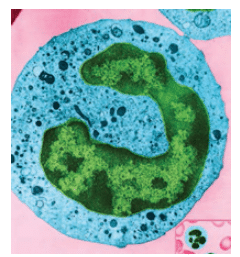
LAP is a disease in which Aggregatibacter actinomycetemcomitans is implicated. Impaired neutrophilic responses appear to accompany neutrophil hypersecretion. Polymorphonuclear leukocytes (PMNs) are one of the host’s first responders to bacterial infection (Figure 2). PMN involvement at the site of infection starts with local complement activation and is followed by intravascular adherence, chemotaxis, phagocytosis, and microbicidal activity (Figure 3).7 PMNs initially adhere to the site of injury, which, in the case of LAP, is caused by Aggregatibacter actinomycetemcomitans infection. Additional PMNs migrate to the site of injury through a process called chemotaxis, which is mediated by complement proteins and other chemotactic molecules.8 The PMNs then engulf the microbes in a process called phagocytosis, and microbicidal activity is carried out either with oxidative or nonoxidative killing. Oxidative killing occurs through a respiratory burst that heightens the production of superoxide, hydrogen peroxide, and hydroxyl radicals.9 Nonoxidative killing occurs via hydrolytic enzymatic activity.
Deficiencies in PMN chemotaxis have been well established in LAP, occurring in 70% to 75% of cases.7,10,11 This diminished response, along with defective phagocytosis,7,12 appear to render hosts susceptible to pathological Aggregatibacter actinomycetemcomitans infections.
PMN functional defects also cause tissue injury in a variety of inflammatory diseases,13 and they appear to exacerbate the severity of LAP. Increased superoxide formation by neutrophils during oxidative killing among patients with LAP has been noted.14,15 Compared with chronic periodontitis, the destruction of periodontal tissues in patients with LAP is accelerated by the excessive production of oxygen-free radicals produced by PMNs.
DIAGNOSIS
In 1999, the AAP described the diagnostic criteria for LAP as “localized first molar/incisor presentation with interproximal attachment loss on at least two permanent teeth, one of which is a first molar, and involving no more than two teeth other than first molars and incisors.”2 Aggressive periodontitis is distinguished from chronic periodontitis by its rapid periodontal destruction accompanied by clinical attachment loss. LAP typically affects young people who are otherwise medically healthy. Individuals with LAP typically exhibit thinner accumulations of plaque biofilm than patients with chronic periodontitis, and there is often little or no subgingival calculus present.16 Aggressive periodontitis also has a strong familial aggregation, which is not seen in chronic periodontitis.
LAP is distinguished from generalized aggressive periodontitis (GAP) by the number of teeth affected, age of onset, and host antibody response. LAP typically occurs around the onset of puberty and has robust serum antibody response, whereas GAP generally affects older patients around age 30 with poor serum antibody response. Both LAP and GAP have rapid rates of progression, but GAP has pronounced episodes of progression.16 These differences make it clear that LAP is not merely a localized version of GAP but an entirely different disease with its own host response and progression rate.
RISK FACTORS
DON W. FAWCETT / SCIENCE SOURCE
Proper risk assessment of patients with LAP is necessary to achieve successful control of disease progression and prevent catastrophic outcomes, such as tooth loss. Possible risk factors for LAP include plaque levels, bacterial composition, genetics, smoking, and stress.
While LAP is a plaque-induced infection, plaque levels or oral hygiene are not strong factors. This is because microbial deposits are not consistent with level of disease, nor is subgingival calculus present.16,17 A study in Brazil, however, found that hygiene and calculus were risk indicators for aggressive periodontitis, although the report did not differentiate between LAP and GAP.18
Although Aggregatibacter actinomycetemcomitans and sometimes Porphyromonas gingivalis have been implicated as etiologic agents of aggressive periodontitis, they alone are not sufficient to cause periodontal disease.19 The presence of Aggregatibacter actinomycetemcomitans does not increase the risk for developing LAP,20 and, therefore, is not characterized as a risk factor or risk indicator.
Familial aggregation has been well established in LAP. It is, however, a complex disease with various pathways and processes that contribute to a clinical presentation, not necessarily following simple Mendelian inheritance. For example, autosomal dominant forms are typically found in the United States, whereas autosomal recessive inheritance is seen in Northern Europe.21 LAP is associated with neutrophil abnormalities and has been linked to chromosome 1q25.21 Genetic deficiencies in neutrophil chemotaxis and phagocytosis, as well as excessive oxygen radical production are implicated in familial inheritance.
While the effects of smoking have been well established in chronic periodontitis, less research has been devoted to aggressive periodontitis, and even less to LAP.17 Some studies indicate that smoking and stress worsen disease progression by influencing cytokine network and modifying microbial flora to be more pathogenic.17,22 However, another report observed worsening disease in the presence of smoking only in GAP.23 The effects of smoking on LAP remain inconclusive due to the limited literature.
GARY CARLSON / SCIENCE SOURCE
TREATMENT
Successful treatment of LAP requires an accurate and prompt diagnosis. Because it is a rare form of periodontal disease, lack of awareness can lead to delays in diagnosis and treatment. Early stages of LAP often go undetected. If LAP is diagnosed at an advanced stage, limited treatment options are available. Early diagnosis is critical for the successful treatment of this rapidly advancing disease.
Once diagnosed, LAP is treated much like other types of periodontitis. Although LAP is not always linked to significant levels of plaque, maintenance of appropriate oral hygiene is fundamental. Multiple long-term studies have demonstrated the importance of professional mechanical plaque removal and consistent self-care in maintaining periodontal health.24 Providing reliable and personalized oral hygiene instruction is an essential part of treatment. Additionally, the need for periodic professional mechanical debridement and follow-up care should be emphasized.
After a proper oral hygiene regimen is implemented, nonsurgical therapy is the next course of action. Little research on the clinical outcomes of nonsurgical therapy on aggressive periodontitis is available. Multiple studies suggest that full-mouth mechanical debridement improves the clinical parameters in patients with LAP up to 3 months.24 These results contradict findings from the 1980s.25–27 The effect of scaling and root planing alone on patients with LAP is inconclusive.
Because LAP does not respond predictably with conventional mechanical debridement therapy alone, clinicians need to seek out adjunctive treatments aimed at potentially improving outcomes. Due to the specific nature of bacterial composition in LAP, chemotherapeutics using systemic/local antibiotic delivery have been explored. More studies are focused on GAP, but limited evidence suggests that adjunctive systemic antibiotics may improve clinical outcomes in LAP. Administration of systemic tetracycline 1 g/day for 28 days adjunctive to scaling and root planing could drastically improve probing depths compared to scaling and root planing alone.28 Complete eradication of Aggregatibacter actinomycetemcomitans was also reported with 8 months of doxycycline treatment following nonsurgical therapy.26 Another study reported that the adjunctive use of metronidazole (600 mg/day for 10 days) with scaling and root planing provided more predictable outcomes than the use of tetracycline.29 A randomized clinical trial that investigated the use of metronidazole (750 mg/day) and amoxicillin (1,500 mg/day) in conjunction with scaling and root planing demonstrated improved effectiveness compared to scaling and root planing alone.30
While the use of local-delivery antimicrobials may be helpful, their effectiveness in treating LAP has been minimally investigated. One study showed that the local administration of either 1% chlorhexidine gel or 40% tetracycline gel did not show additional improvement compared to controls after 12 months.31 Even in conjunction with systemic antibacterial therapy, the benefits of local antimicrobials are not substantial when cost is considered.32 Clinicians should use their own experience and judgment when deciding whether to implement local antimicrobials in the treatment of LAP.
Often, the diagnosis of LAP is made at an advanced stage of the disease. Therefore, after initial nonsurgical therapy is completed, residual and unresolved periodontal pockets may necessitate definitive surgical therapy. Unresolved pockets can be treated by resective surgery or regenerative surgery. Several studies (although with small sample sizes) demonstrated the effectiveness of resective surgeries. Christersson et al33 demonstrated that scaling and root planing alone did not effectively suppress Aggregatibacter actinomycetemcomitans in periodontal pockets. In comparison, modified Widman flap surgery group showed such suppression, and all clinical parameters were improved. In two other studies, patients with LAP who received the modified Widman flap surgery experienced significant clinical attachment gains and Aggregatibacter actinomycetemcomitans was eradicated for at least 12 months—and, in some patients, for up to 5 years post-surgery.34,35 Systemic antibiotics can be added as adjunctive treatment to surgery, and favorable outcomes have been reported.36
Unresolved periodontal pockets occasionally may lead to vertical three-walled intrabony defects, which should be treated with regenerative therapy instead of resective surgery. Guided tissue regeneration using bone grafting and membrane placement is the most predictable modality to treat intrabony defects. Sirirat et al37 reported that the use of nonresorbable membrane with osseous surgery resulted in significant bone and attachment gain compared to osseous surgery alone after 12 months of follow-up care. Bioactive modifiers, such as growth factors and differentiation factors, have also been investigated with successful outcomes reported.38 However, evidence on the efficacy of these modifiers in the treatment of patients with LAP is limited. Clinicians should make the decision to use bioactive modifiers in this patient population on a case-by-case basis using their best clinical judgment.
If LAP is diagnosed too late, severe bone loss may lead to tooth loss. Implant therapy is often considered the treatment of choice for edentulous sites. However, no literature is available on the success of implant therapy in patients with a history of LAP. One 10-year prospective cohort study on implant therapy in patients with GAP found that it was successful in partially edentulous sites.39 Therefore, tooth extraction followed by implant therapy may be a rational approach for patients who display signs of LAP.
For patients with LAP, the importance of supportive periodontal therapy or periodontal maintenance cannot be overemphasized.40 Although GAP has a much greater risk (35 fold) of recurrence than LAP, patients with LAP should be closely monitored as delay in detection may result in poor outcomes. A longitudinal study showed that LAP seems to stabilize once the supportive periodontal therapy phase is entered after initial treatment is complete. Periodontal maintenance is the key to long-term stability of the disease process in this patient population.25
PREVENTION STRATEGIES
LAP, like the more common chronic periodontitis, is a dental plaque-induced periodontal disease. Prevention should start with identifying and controlling modifiable risk factors, such as smoking cessation (when applicable); optimal plaque control; and supporting patient compliance with needed periodontal therapy. Although the amount of biofilm and calculus present is not strongly correlated with the level of disease, infection by Aggregatibacter actinomycetemcomitans is necessary to cause disease. Thus, proper self-care with periodic hygiene visits in which oral hygiene instructions are provided is crucial to preventing LAP. Patients should be advised to brush with an antimicrobial toothpaste, perform interdental cleaning, and use an antimicrobial mouthrinse to control bacteria levels.
Although genetic risk factors cannot be modified, documenting family history of LAP is essential to identifying patients who are at increased risk of the disease. Due to LAP’s rapid progression, high-risk patients should be closely monitored and educated about the disease so that prompt diagnosis and treatment can be provided, if needed.
Compliance with an effective self-care regimen and keeping periodontal maintenance appointments are key to preventing LAP. Periodontal maintenance visits should include periodic monitoring of health status and continued education on self-care. There are no well-established official recommendations regarding recare intervals for patients with LAP, so this decision is made based on the clinician’s judgment. Intervals of 3 months to 4 months seem reasonable due to the aggressive nature of the disease. Stressing compliance is a modifiable factor that clinicians should keep in mind, as compliance rates for patients in periodontal maintenance hover around 40%.41,42 Appointment reminders and behavior modification techniques, such as explaining why periodontal maintenance is important and consistent motivation on adhering to self-care regimens, may be helpful in improving compliance rates.42
CONCLUSION
LAP is a relatively rare form of periodontal disease that, if ignored, can lead to tooth loss. Accurate, early diagnosis and careful identification of modifiable risk factors are integral to successful treatment. Once it is diagnosed, LAP can be treated in a manner similar to chronic periodontitis with strategies such as nonsurgical therapy, chemotherapeutics, and surgery. After the disease is stabilized, self-care and supportive periodontal therapy are critical to preventing disease recurrence. Periodontal surgery—to include tactics aimed at reducing pocket probing depths via resection, regeneration, or a combination of both—may also be helpful. ?
ACKNOWLEDGEMENTS
The author would like to thank Cliff Lee and Sohyun Park, DMD, for their assistance with this manuscript.
References
- Baer PN. The case for periodontosis as a clinical entity. J Periodontol. 1971;42:516–520.
- Armitage GC. Development of a classification system for periodontal diseases and conditions.Ann Periodontol. 1999;4:1–6.
- Albandar JM, Tinoco EM. Global epidemiology of periodontal diseases in children and youngpersons. Periodontol 2000. 2002;29:153–176.
- Oh TJ, Eber R, Wang HL. Periodontal diseases in the child and adolescent. J Clin Periodontol.2002;29:400–410.
- Zambon JJ, Christersson LA, Genco RJ. Diagnosis and treatment of localized juvenileperiodontitis. J Am Dent Assoc. 1986;113:295–299.
- Krill DB, Fry HR. Treatment of localized juvenile periodontitis (periodontosis). A review.J Periodontol. 1987;58:1–8.
- Van Dyke TE, Zinney W, Winkel K, Taufiq A, Offenbacher S, Arnold RR. Neutrophil function inlocalized juvenile periodontitis. Phagocytosis, superoxide production and specific granule release.J Periodontol. 1986;57:703–708.
- Dennison DK, Van Dyke TE. The acute inflammatory response and the role of phagocytic cellsin periodontal health and disease. Periodontol 2000. 1997;14:54–78.
- Badwey JA, Karnovsky ML. Active oxygen species and the functions of phagocytic leukocytes.Annu Rev Biochem. 1980;49:695–726.
- Van Dyke TE, Schweinebraten M, Cianciola LJ, Offenbacher S, Genco RJ. Neutrophil chemotaxisin families with localized juvenile periodontitis. J Periodontal Res. 1985;20:503–514.
- Van Dyke TE, Horoszewicz HU, Cianciola LJ, Genco RJ. Neutrophil chemotaxis dysfunction inhuman periodontitis. Infect Immun. 1980;27:124–132.
- Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE. Impaired phagocytosisin localized aggressive periodontitis: rescue by Resolvin E1. PloS One. 2011;6:e24422.
- Weiss SJ. Tissue destruction by neutrophils. New Eng J Med. 1989;320:365–376.
- Shapira L, Borinski R, Sela MN, Soskolne A. Superoxide formation and chemiluminescence ofperipheral polymorphonuclear leukocytes in rapidly progressive periodontitis patients. J ClinPeriodontol. 1991;18:44–48.
- Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal diseasepathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74:66–75.
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol2000. 2004;34:9–21.
- Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronicperiodontitis and aggressive periodontitis. Periodontol 2000. 2010;53:138–153.
- Susin C, Albandar JM. Aggressive periodontitis in an urban population in southern Brazil.J Periodontol. 2005;76:468–475.
- Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7.
- Buchmann R, Muller RF, Heinecke A, Lange DE. Actinobacillus actinomycetemcomitans indestructive periodontal disease. Three-year follow-up results. J Periodontol. 2000;71:444–453.
- Li Y, Xu L, Hasturk H, Kantarci A, DePalma SR, Van Dyke TE. Localized aggressive periodontitisis linked to human chromosome 1q25. Hum Genet. 2004;114:291–297.
- Kamma JJ, Giannopoulou C, Vasdekis VG, Mombelli A. Cytokine profile in gingival crevicularfluid of aggressive periodontitis: influence of smoking and stress. J Clin Periodontol.2004;31:894–902.
- Schenkein HA, Gunsolley JC, Koertge TE, Schenkein JG, Tew JG. Smoking and its effects on earlyonsetperiodontitis. J Am Dent Assoc. 1995;126:1107–1113.
- Axelsson P, Nystrom B, Lindhe J. The long-term effect of a plaque control program on toothmortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J ClinPeriodontol. 2004;31:749–757.
- Gunsolley JC, Califano JV, Koertge TE, Burmeister JA, Cooper LC, Schenkein HA. Longitudinalassessment of early onset periodontitis. J Periodontol. 1995;66:321–328.
- Saxen L, Asikainen S, Kanervo A, Kari K, Jousimies-Somer H. The long-term efficacy of systemicdoxycycline medication in the treatment of localized juvenile periodontitis. Arch Oral Biol.1990;35(Suppl):227S–229S.
- Saxen L, Asikainen S, Sandholm L, Kari K. Treatment of juvenile periodontitis withoutantibiotics. A follow-up study. J Clin Periodontol. 1986;13:714–719.
- Kornman KS, Robertson PB. Clinical and microbiological evaluation of therapy for juvenileperiodontitis. J Periodontol. 1985;56:443–446.
- Saxen L, Asikainen S. Metronidazole in the treatment of localized juvenile periodontitis. J ClinPeriodontol. 1993;20:166–171.
- Tinoco EM, Beldi MI, Campedelli F, et al. Clinical and microbiological effects of adjunctiveantibiotics in treatment of localized juvenile periodontitis. A controlled clinical trial. J Periodontol.1998;69:1355–1363.
- Unsal E, Walsh TF, Akkaya M. The effect of a single application of subgingival antimicrobial ormechanical therapy on the clinical parameters of juvenile periodontitis. J Periodontol.1995;66:47–51.
- Purucker P, Mertes H, Goodson JM, Bernimoulin JP. Local versus systemic adjunctive antibiotictherapy in 28 patients with generalized aggressive periodontitis. J Periodontol.2001;72:1241–1245.
- Christersson LA, Zambon JJ. Suppression of subgingival Actinobacillus actinomycetemcomitansin localized juvenile periodontitis by systemic tetracycline. J Clin Periodontol. 1993;20:395–401.
- Lindhe J, Liljenberg B. Treatment of localized juvenile periodontitis. Results after 5 years. J ClinPeriodontol. 1984;11:399–410.
- Mandell RL, Socransky SS. Microbiological and clinical effects of surgery plus doxycycline onjuvenile periodontitis. J Periodontol. 1988;59:373–379.
- Buchmann R, Nunn ME, Van Dyke TE, Lange DE. Aggressive periodontitis: 5-year follow-up oftreatment. J Periodontol. 2002;73:675–683.
- Sirirat M, Kasetsuwan J, Jeffcoat MK. Comparison between 2 surgical techniques for thetreatment of early-onset periodontitis. J Periodontol. 1996;67:603–607.
- Gupta G. Clinical and radiographic evaluation of intra-bony defects in localized aggressiveperiodontitis patients with platelet rich plasma/hydroxyapatite graft: A comparative controlledclinical trial. Contemp Clin Dent. 2014;5:445–451.
- Mengel R, Behle M, Flores-de-Jacoby L. Osseointegrated implants in subjects treated forgeneralized aggressive periodontitis: 10-year results of a prospective, long-term cohort study.J Periodontol. 2007;78:2229–2237.
- Kamma JJ, Baehni PC. Five-year maintenance follow-up of early-onset periodontitis patients.J Clin Periodontol. 2003;30:562–572.
- Wilson TG Jr, Glover ME, Schoen J, Baus C, Jacobs T. Compliance with maintenance therapy in aprivate periodontal practice. J Periodontol. 1984;55:468–473.
- Wilson TG Jr, Hale S, Temple R. The results of efforts to improve compliance with supportiveperiodontal treatment in a private practice. J Periodontol. 1993;64:311–314.
From Dimensions of Dental Hygiene. February 2016;14(02):53–58.



