
Partner With Your Patients
How to keep the remineralization/demineralization process in balance.
Remineralization is a dynamic interaction between the biochemical properties of the oral cavity, enamel, and dentin that is changing the way dental lesions are being treated. Remineralization relies heavily on the partnership between clinicians and patients for positive outcomes. Dental professionals must consider the dynamics of remineralization when determining patients’ risk factors for dental lesions and partner with patients to manage and enhance the repair process.
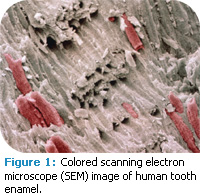
Enamel is a highly calcified tissue that is among the hardest in vertebrates (Figure 1). It consists of 96% calcium hydroxyapatite (mineral), 3% water, and 1% organic material. The highest concentration of mineral exists near the enamel surface.1 This high mineral content provides protection against mechanical damage. However, enamel is brittle and relies on dentin for support. It is vulnerable to assaults of varying etiology and pathogenesis such as dental caries, attrition, abrasion, abfraction, and erosion, and cannot renew itself because its formative cells are lost after eruption. To compensate for this disadvantage, enamel is capable of limited repair through changes in mineralization. After demineralization dissolves the most soluble material, remineralization can replace the lost mineral with a less soluble apatite.
REMINERALIZATION
 Remineralization is a natural repair reaction that relies on calcium and phosphate ions, assisted by fluoride, to build a new surface that is more highly mineralized and less soluble in the presence of acids.2 The repair process is an ongoing and stabilizing process of mineralization that alternates between the demineralization of enamel and the remineralization of less mineralized enamel. It is highly dependent on equilibrium between pathological and protective factors. Once this balance is lost, the balance may tip toward health or disease.
Remineralization is a natural repair reaction that relies on calcium and phosphate ions, assisted by fluoride, to build a new surface that is more highly mineralized and less soluble in the presence of acids.2 The repair process is an ongoing and stabilizing process of mineralization that alternates between the demineralization of enamel and the remineralization of less mineralized enamel. It is highly dependent on equilibrium between pathological and protective factors. Once this balance is lost, the balance may tip toward health or disease.
To restore the natural equilibrium, either remineralization must be enhanced or demineralization slowed. Early enamel lesions have a potential for remineralization with an increased resistance to further acid challenge, particularly with the use of enhanced remineralization treatments and management of certain biological factors.3
ROLE OF SALIVA
Saliva is the leading biological factor in maintaining the balance between mineral loss and gain. It is nature’s primary defense system for the oral cavity. It aids in buffering acids as well as contributes calcium, phosphate, and fluoride ions to maintain enamel integrity. Saliva not only provides everything needed for remineralization but also plays an important role in the digestion and clearance of food, while also providing protective antimicrobial agents.
The protective nature and remineralizing effect of salivary biofilm appears to function similarly in dental erosion and caries disease.4,5 Glycoproteins from saliva adsorb onto the tooth structure to form the protective pellicle layer and phosphoproteins regulate calcium saturation of the saliva. Pellicle formation, as early as the first 3 minutes of formation, provides protection to enamel after an acid challenge and imparts significant resistance to enamel demineralization. There is evidence that saliva plays a reparative role after an erosive challenge on the tooth structure by providing organic and mineral material to fill in microscopic defects on the tooth structure. As such, it is important to find ways to increase salivary flow rate.6 Sorbitol- and xylitol-sweetened chewing gum increase salivary flow and protect enamel following a sucrose challenge (Figures 2 and 3).7,8 The chewing action stimulates salivary flow, increases the buffering capacity of saliva, and neutralizes the drop in plaque pH.8 Demineralization appears to halt with initiation of chewing and remains stable for at least 30 minutes. Additionally, consistent use of sugar alcohol-sweetened gum acts to “starve” cariogenic microorganisms by replacing the source of sucrose. There is a resultant slowing down in reproduction of Streptococcus mutans and a decrease in the overall S. mutans count.9-11
REMINERALIZING OF DENTAL LESIONS UP CLOSE
Dental caries is an infectious disease caused by acid-producing bacteria that metabolize dietary fermentable carbohydrates (Figure 4). The first visible sign of caries that can be seen with the naked eye is a white spot lesion. The lesion is completely dependent on the location and specific environmental conditions of the overlaying biofilm, and permanent removal of the biofilm should, in theory, put an end to the demineralization process of the lesion.12 However, Manji et al suggest that since biofilm is ever present intraorally and always metabolically active, caries is an equally ubiquitous phenomenon that can only be controlled as long as it does not become clinically apparent through cavitation.13 Additionally, Koulourides demonstrated in vitro that saliva remineralizes incipient enamel lesions and that low level amounts of fluoride accelerate the process.14 Partially demineralized enamel lesions can be remineralized to approximate the original size in vitro.15 However, if the surface layer is lost completely, as in erosion, remineralization techniques will not result in the return of the surface layer. Since most remineralizing occurs at the surface, research is underway to provide better access of macromolecular reparative complexes (minerals) to subsurface areas. Controlled acid etching of remineralized surface lesions is being investigated to enhance the remineralization potential of both dental caries lesions and dental erosion.16
In contrast to dental carious lesions, where initial mineral loss is mostly subsurface, dental erosion results in mineral loss of the outer few micrometers of the enamel surface by direct contact with acids unrelated to microbial involvement. The typical clinical appearance of erosion is a smooth silky-glazed or dull enamel surface.17 Erosion usually appears on the lingual or facial surfaces depending on the cause. Appearance on the posterior teeth is possible, however, and may note the presence of gastroesophageal reflux disease.18 In erosive lesions, hard surface dental structures are chemically etched away from the tooth surface through nonbacterial chemical assaults involving acidic substances, such as gastric acids from chronic vomiting and reflux disease, carbonated beverages, citrus fruits, salad dressing, herbal teas, and acidic medicines.17 External sources common to industrial or environmental settings include acid fumes and aerosol found in battery and fertilizer factories. The erosive strength of a substance depends not only on pH value and type of acid but also on the buffering capacity, calcium chelation properties, mineral content, and ability to adhere to enamel.18
Erosive lesions progress in an incremental pattern of decalcification. Eventually the acids diffuse into the deeper areas of enamel and further dissolve minerals under the surface, creating a concavity whose width exceeds its depth.19,20 An acid attack of this nature, whether from extrinsic or intrinsic sources, leads to a softened, partly demineralized tooth surface that is more susceptible to the effects of mechanical abrasion (toothbrushing) and attrition. In patients at high risk of dental erosion, toothbrushing should be avoided immediately after consuming erosive food and beverages, such as sport drinks, fruit juices, and herbal teas.21 Instead, patients should use a fluoride-containing mouth rinse or sodium bicarbonate solution, rinse with water, or consume milk, cheese, or sugar-free yogurt. Erosion, though lacking a bacterial component, is similar to caries because it is a process of repeated demineralization caused by an imbalance in the ecological and chemical equilibrium, and is multifactorial in nature.
THERAPEUTIC AGENTS
Salivary components and therapeutic agents act cooperatively in the enamel repair continuum. Therapeutic agents mimic and enhance the effects of oral biochemistry. Several calcium phosphate technologies are emerging with varying amounts of research. A reactive and soluble calcium phosphate, amorphous calcium phosphate (ACP), is a compound that releases calcium and phosphate ions to change to apatite and aid in remineralization when in contact with saliva. ACP forms on enamel within dentinal tubules and offers a reservoir of calcium and phosphate ions in the saliva.22,23
Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) regulates the behavior of calcium phosphate and has a stabilizing effect. Products containing CPP-ACP or Recaldent™ are derived from the milk protein casein. CPP carries calcium and phosphate ions in the form of ACP. When CPP-ACP is added to the oral cavity, it binds to the enamel and biofilm. This incorporation into the salivary pellicle can reduce the adherence of plaque bacteria. It can deliver calcium and phosphate ions to areas that are demineralized and provide better remineralization by protecting tooth enamel against erosive acid challenges.24
Arginine, a remineralizing calcium-based paste, was introduced in the 1990s. It can be incorporated into multiple delivery systems including toothpaste and prophy paste. Arginine can encourage remineralization of initial carious lesions by providing calcium from calcium carbonate, which is activated when the plaque pH begins to decrease.25,26 Calcium sodium phosphosilicate (NovaMin®) is composed of calcium, phosphorous, sodium, and silica and interacts with saliva to release Ca2+, P5+, and Na+. First, the Na+ buffers the acid and then the charged Ca2+ and P5 ions saturate saliva to create a new layer of hydroxyapatite that fills demineralized lesions.23,27
ORAL HYGIENE AND DIETARY PRACTICES
Enamel resistance and biochemical properties of oral ecology are not the only modulating factors in the remineralization and demineralization of tooth enamel. Dietary practices and behaviors relative to oral hygiene play an important role in the proper maintenance of the repair/ destruction continuum. Although caries disease and erosion have different etiologies and pathogenesis, they both tip the scales in favor of acid demineralization. There is evidence that they are influenced by diet and oral hygiene.28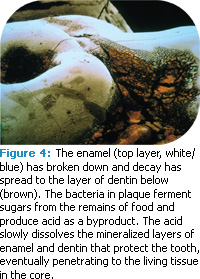
The new evidence regarding mineralization potential of dental caries and erosive lesions has led to paradigm changes in their management and treatment. The shift is from a surgical approach to a noninvasive approach that focuses on management of dental biofilm. This new approach addresses the risk factors responsible for the disease, relies on early intervention and manipulation of the dental biofilm, and enhances the inherent oral biological repair system.
A balance between pathological and preventive factors will determine the remineralization potential of dental caries and erosion, and oral hygiene practices alone (such as brushing and flossing) will not arrest them.29,30 Fluoride inhibits demineralization, boosts remineralization, and can inhibit cariogenic bacteria. Fluoride enhances remineralization of partially dissolved enamel and dentin crystals by combining with calcium and phosphate that comes primarily from saliva. When fluoride is deposited into the enamel structure, fluorapatite replaces hydroxyapatite that produces a stronger and larger apatite crystal. This crystal is more resistant to acids and will try to inhibit demineralization. Topical fluorides can be applied through toothpastes, prophy pastes, gels, varnishes, foam, and mouth rinses.
THE FUTURE OF CLINICAL PRACTICE AND RESEARCH
Possibilities exist to intervene in the dynamic process to arrest or reverse the progress of dental lesions since caries and erosion involve cycles of demineralization and remineralization. Remineralization relies on the enhancement of certain protective biological factors: salivary flow in concert with calcium and phosphate ions assisted by fluorides to rebuild a new surface on existing crystal remnants that remain after demineralization. In the realm of these possibilities, increasing the mineralization potential of enamel may result in a more conservative, nonsurgical approach to inhibiting and preventing lesion progression, as well as an imperative to broaden the search for new and better management tools. Past research examines remineralization of superficial lesions (up to 100 ? m). A growing body of evidence suggests that noncavitated carious lesions that extend histologically into the dentin can be remineralized under optimal conditions.31 Remineralization throughout the depth of the lesion and into the dentin is possible.32
CONCLUSION
Dental professionals need to identify the oral ecological imbalance to facilitate biological changes in the oral cavity. This can be accomplished by recognizing patients at risk of erosive lesions and promoting remineralization. Dental lesions are never cured but their healing potential can be maximized. It is an ongoing process that must be monitored closely. Dietary and lifestyle patterns that contribute to etiology and pathogenicity of dental lesions need to be managed, while behaviors and therapeutic modalities that enhance remineralization and repair need to be employed. This new protocol for treatment of dental caries and erosive lesions represents a partnership with patients that must be continued throughout their lifetimes.
FIGURE 1: ANDREW SYRED / PHOTO RESEARCHERS, INC; FIGURES 2-3: SIDNEY MOULDS / PHOTO RESEARCHERS, INC.
REFERENCES
- Øgaard B. The cariostatic mechanism of fluoride. Compend Contin Educ Dent. 1999;20 (1 Suppl):10-17.
- Featherstone JD. Dental caries: a dynamic disease process. Aust Dent J. 2008;53:286-291.
- Lata S, Varghese NO, Varughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho-peptide on enamel lesions: An in vitro comparative evaluation. J Conserv Dent. 2010; 13:42-46.
- Hannig M, Hess NJ, Hoth-Hannig W, De Vrese M. Influence of salivary pellicle formation time on enamel demineralization—an in situ pilot study. Clin Oral Investig. 2003;7:158-161.
- Hall AF, Buchanan CA, Millett DT, Creanor SL, Strang R, Foye RH. The effect of saliva on enamel and dentine erosion. J Dent. 1999; 27:333-339.
From Dimensions of Dental Hygiene. March 2011; 9(3): 58-60, 62.

