The Science of Polishing
Appropriate technique and advances in traditional polishing.
Whether or not dental hygienists support the theory of selective polishing, polishing is an integral dental procedure.1,2 While the process of polishing appears to be straightforward and even mundane, polishing involves several complex issues, many of which are interdependent. Polishing is inherent in dental hygiene practice and can cause unnecessary harm if not performed correctly.3 The polishing of teeth and dental restorations is important for patient satisfaction and for success in the prevention and treatment of oral disease.
The American Academy of Periodontology defines oral prophylaxis as the “removal of plaque, calculus, and stain from exposed and unexposed surfaces of the teeth by scaling and polishing as a preventive measure for the control of local irritational factors.”4 A white paper on oral prophylaxis published by the American Dental Hygienists’ Association states “the oral prophylaxis should consist of supragingival and subgingival removal of plaque, calculus, and stain.”5
There are several reasons for integrating polishing in an oral prophylaxis.6 Polishing produces smooth surfaces on the teeth and restorations, thereby reducing the adherence of oral accretions: dental plaque, extrinsic stains, and calculus. From the patient’s perspective, the removal of extrinsic stains from teeth and restorative materials that can enhance esthetics is desirable. Each year millions of dollars are spent on procedures to whiten and brighten smiles, which emphasizes the fact that an attractive smile is important to consumers. Patients expect to have their teeth polished when they have their teeth “cleaned” and they expect to have that “smooth” feeling when leaving the oral prophylaxis appointment. Dental hygienists are responding to patients’ expectations of having their teeth polished as part of an oral prophylaxis, which is demonstrated by the amount of polishing products sold by dental manufacturers and distributors each year.7
Mechanisms of Polishing
In abrasive science, mechanisms of polishing that use abrasive particles are part of tribiology, a discipline associated with physics, chemistry, materials science, and surface-contact engineering.8 Specifically, tribiology is the science of interacting surfaces in relative motion; it incorporates the study and application of the principles of friction, lubrication, and wear. From a material science perspective, polishing is intended to produce intentional, selective, and controlled wear. Within the science of tribiology, polishing can be considered two-body abrasion or three-body abrasion.9-11 In twobody abrasive polishing, the bound particles are solidly fixed to a substrate. Two-body abrasive products have rubber cups impregnated with abrasive agents and thus do not require polishing paste. Dental hygienists primarily use three-body abrasion, in which loose abrasives (the polishing paste abrasive particles) move in the interface space between the surface being polished and the polishing application device.
Six factors largely determine the rate of abrasion when polishing: speed, pressure, quantity of paste applied, shape of the abrasive particles, size of the abrasive particle, and hardness of the abrasive particle.9,12,13 Speed and pressure, which are operator controlled, increase the rate of abrasion and create heat, which can initiate or worsen dentinal hypersensitivity and should be kept to a minimum. When speed is increased, pressure should be decreased and vice versa. The shape of the abrasive particles also affects the rate of abrasion. Round or spherically-shaped particles abrade slower than sharp, irregular-shaped particles.
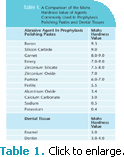 Hardness of the abrasive particle is profoundly important to the rate of polishing.14 The hardness, size, and shape of particles are what make them abrasive. Abrasive particles must be harder than the surface or material being polished; harder particles abrade faster.9,11 When comparing abrasives to tooth structures, many of the abrasives used in prophylaxis polishing pastes are 10 times harder than the tooth structure to which they are applied.15 Table 1 provides a comparison of the Mohs Hardness Value of dental tissues to agents commonly used in prophylaxis polishing pastes.13 The Mohs Hardness Scale is a relative measure of mineral hardness (resistance to abrasion, not indentation). It is a comparative index, not a linear scale.
Hardness of the abrasive particle is profoundly important to the rate of polishing.14 The hardness, size, and shape of particles are what make them abrasive. Abrasive particles must be harder than the surface or material being polished; harder particles abrade faster.9,11 When comparing abrasives to tooth structures, many of the abrasives used in prophylaxis polishing pastes are 10 times harder than the tooth structure to which they are applied.15 Table 1 provides a comparison of the Mohs Hardness Value of dental tissues to agents commonly used in prophylaxis polishing pastes.13 The Mohs Hardness Scale is a relative measure of mineral hardness (resistance to abrasion, not indentation). It is a comparative index, not a linear scale.
Prophylaxis polishing agents are available in two basic forms: dry powders, also referred to as flours that must be mixed with a liquid ( water, fluoride, or mouthrinse) and commercially prepared polishing pastes that are available in bulk or individual unit doses. Dry powders or flours are not graded according to grit, rather they are graded in order of increasing fineness: F, FF, and FFF.15 Powders or flours with no wetting agent represent the greatest quantity of abrasives that can be applied per unit of time and they create excessive heat. Therefore, the use of dry abrasives or powder on a dry polishing cup is contraindicated due to the potential for thermal injury to natural teeth.
The grit of commercially prepared polishing pastes is graded from fine to coarse, based on a standard sieve through which the particles pass.16 The types of abrasive particles used in polishing pastes vary among the commercial varieties and from one grit size to another, yet there is no industry standard to define what these terms mean or what size the abrasive particle must be. The types of abrasive particles used in commercial prophylaxis polishing pastes include flour of pumice, aluminum oxide (alumina), silicon carbide, aluminum silicate, silicon dioxide, carbide compounds, garnet, feldspar, zirconium silicate, zirconium oxide, boron, and calcium carbonate.13,14 Others include emery, perlite, and silica.17
Commercially prepared prophylaxis polishing pastes combine abrasives with a binder, humectants, coloring agent, preservatives, and flavoring agents.13 Manufacturers generally do not disclose the amount of ingredients in their polishing pastes because the information is proprietary. However, it is general knowledge that pumice and glycerin are the most commonly used ingredients in commercially prepared polishing pastes. Some commercially prepared polishing pastes contain fluoride. Fluoride in prophylaxis polishing pastes is not a replacement for a fluoride treatment.6,13,18,19 Insurance companies will not reimburse a fluoride treatment that is billed when the only fluoride applied is in the form of fluoride-containing prophylaxis polishing pastes.
At this time, the Food and Drug Administration (FDA) does not regulate polishing pastes.20 The FDA does not recognize or designate any oral prophylaxis paste as containing a drug, and therapeutic additives are recognized by the FDA as secondary components.
Because tooth polishing is an inherent procedure in oral health care, dental hygienists need to select the prophylaxis polishing paste based on the individual patient’s needs. The treatment plan for polishing should be based on caries risk (presence of demineralization), presence of exposed dentin, erosion and/or abrasion, and the type of restorations present and type of stain present. If patients do not require stain removal, yet have the expectation of having their teeth “polished,” an excellent alternative is a cleaning agent.1 Cleaning agents do not contain abrasive particles, therefore, they do not abrade the surfaces of teeth or restorative materials. Cleaning agents havefl at, round particles yet still produce a high luster. The most readily available cleaning agent is made of feldspar, a naturally occurring mineral that covers 60% of the earth’s crust. Feldspar particles are made of alkali, a compound of sodium, potassium, and calcium aluminosilicates. This cleaning agent is formulated into a powder and can be mixed with sodium fluoride or water to apply as a paste.
To create the desired outcome of a highly polished surface, the polishing process should begin with the application of the coarsest grit abrasive necessary to meet the patient’s needs. This process is followed by the application of a medium grit abrasive and ends with the application of a fine grit particle. The tooth surfaces to be polished should be approached with a series of finer and finer abrasives, until the microscopic scratches are smaller than the wavelength of visible light (<.05 µm).13 When scratches this size are created, the surface appears smooth and shiny—the smaller the scratches, the more gloss and luster the surface. Mixing larger abrasive particles with smaller particles will not produce the desired shiny surface because the particle sizes will mix. Each size grit of the abrasive agents must be applied separately. Therefore, to prevent each grit size from being contaminated with the previous size grit, the rubber cup must be replaced between applications.13 During the polishing procedure, it is important to frequently renew the polishing paste in the polishing cup because the paste may dry as the binders are expressed during use, making the paste more abrasive.
Polishing Reality
Unfortunately, many dental hygienists use whatever type of polishing paste is available on every patient, regardless of the grit size. Even worse is the fact that some dental hygienists subscribe to the “coarse grit theory.” The premise for this ill-advised idea is that the use of the coarsest polishing paste available will remove the heaviest amounts of stain as well as the lightest amounts, thus saving time. Providers who polish in this fashion ignore the professionally recommended method of using the polishing grits in a progression of coarse, medium, and fine applications, which is supported by well-established science and is applied not only in health care but in mechanical polishing procedures in a variety of industries. In an ideal setting the progression from coarse to fine paste is best. In clinical practice, if a dental hygienist is using medium grit paste it’s best to follow with fine grit. If fine grit is used routinely, medium or coarse pastes are only needed in situations of heavy stain.
Proof of the widespread use of the “coarse pumice theory” lies in the published sales of coarse grit as the leading selling brand of polishing paste; 80% of polishing paste sales are in coarse grit and 10% are in medium grit.7 Coarse grit polishing pastes may remove stain quickly, but at a price. Coarse grit polishing pastes can produce hypersensitivity, rough tooth surfaces, and accelerate staining and the retention of dental plaque and calculus. The surface characterization of esthetic dental restorations can be highly damaged by polishing pastes.21-23
New Options and Available Evidence
For many years, the most notable advancement in traditional polishing was the introduction of prophy pastes in unit-dose cups. Since then, new formulations of commercial polishing pastes have been added to the polishing armamentarium.
For more than a decade, commercial polishing pastes that contain perlite25 as an abrasive ingredient have been available. Prophy pastes containing perlite make claims that the abrasive particles break down and become less abrasive under pressure. Scientific evidence supports the fact that the abrasive agents in these products do break down under load (pressure). However, scientific evidence supports the fact that most abrasives in polishing pastes break down under pressure.
Amorphous calcium phosphate (ACP) products that include a polishing paste claim to remineralize enamel subsurface carious lesions. These products are missing a body of research in vivo. The current research exists only in vitro. Three scientific questions need to be addressed: Is it possible to burnish an ingredient such as ACP into enamel with a polishing product that is abrasive and meant to remove stain? What are the true benefits of ACP or similar products such as casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) over the known remineralization properties of fluoride? Why has fluoride been added to some of these ACP and CPP-ACP products?
Polishing paste with calcium sodium phosphosilicate is a new development. Calcium sodium phosphosilicate is a bioactive glass that releases calcium and phosphorus ions rapidly26 and is currently being incorporated into other dental products. Scientific clinical research is not available to support the claim that this product immediately relieves dentinal hypersensitivity. Some in vitro studies of ACP-, CPP-ACP-, and calcium sodium phosphosilicate-containing products do indicate clinical promise, however, the lack of in vivo research to date is the matter of concern.
It will be a leap forward if the additives to polishing pastes can remineralize carious lesions and immediately relieve hypersensitivity on a long-term basis. There is no doubt that polishing pastes are going through a period of renewal as manufacturers are looking for ways to add remarkably active ingredients to such an inexpensive and easy delivery system. It is challenging, however, for manufacturers to add these novel ingredients while retaining the expected performance of polishing pastes. Hopefully, the future will bring about this much-needed research and the introduction of new products.
References
- Barnes CM. Protocol for polishing. Dimensions of Dental Hygiene. 2004;2(6):26-32.
- Beebe SN. To polish or not to polish? Under standing the principle and pros and cons of selective polishing. Dimensions of Dental Hygiene. 2009;7(3):32,35.
- Guideline on the role of dental prophylaxis in pediatric dentistry. Pediatr Dent. 2007-2008;29 (7 Suppl):1-271.
- Glossary of Periodontal Terms. 3rd ed. Chicago: American Academy of Periodontology; 1992:40.
- American Dental Hygienists’ Association. Position Paper on the Oral Prophylaxis. Available at: www.adha.org/profissues/prophylaxis.htm. Accessed October 20, 2009.
- Daniel SJ, Harfst SA, Wilder RS. Mosby’s Dental Hygiene Concepts, Cases, and Competencies. 2nd ed. St. Louis: Mosby Elsevier; 2008:600-622.
- Strategic Data Marketing. Available at: www.sdmdata.com. Accessed October 21, 2009.
- Jeffries SR. Abrasive finishing and polishing in restorative dentistry: a state of the art review. Dent Clin N Am. 2007;51:379-397.
- Hutchings IM. Abrasion process in wear and manufacturing. Proceedings of the Institution of Mechanical Engineers. Part J: Journal of Engineering Tribology. 2002;216:55-62.
- Remond G, Nockolds C, Phillips M, et al. Implications of polishing techniques in quantitative x- ray microanalysis. J Res Natl Inst Stand Technol. 2002;107:639-662.
- Williams JA. Wear and wear particles—some fundamentals. Tribiology International. 2005;38:863-870.
- O’Brien WJ. Dental Materials and Their Selection. 3rd ed. Carol Stream, Ill: Quintessence; 2002:156-160.
- Wilkins EM. Clinical Practice of the Dental Hygienist. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2009:727-740.
- Ferracane JL. Materials in Dentistry Principles and Applications. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2001:288-289.
- Pumice Typical Screen Analysis of Grades. Available at: www.groshea.com/crminerals/screen.html. Accessed October 20, 2009.
- Abrasive Grains 101, Characterization of Abrasives. Available at: www.uama.org/Abrasives101/101Character.html. Accessed October 20, 2009.
- Basic Facts About Perlite. Available at: www.perlite.net/redco/basic.htm. Accessed October 20, 2009.
- Koch G, Petersson LG, Johnson G. Abrasive effect and fluoride uptake from polishing and prophylactic pastes. Sven Tandlak Tidskr. 1975;68:1-7. Swedish.
- Featherstone JBD. Elements of a successful adult caries preventive program. Compendium. 2001;8:1-9.
- U.S. Food and Drug Administration. Jurisdictional update: dental prophylaxis pastes with drug components. Available at: www.fda.gov/CombinationProducts/JurisdictionalInformation/JurisdictionalUpdates/ucm106560.htm. Accessed October 20, 2009.
- Barnes CM, Covey D, Johnson WW, St. Germain HA. Maintaining restorations for senior dental patients. Journal of Practical Hygiene. 2003;12:25.
- Barnes, CM, Covey DA, Walker MP, Johnson WW. Essential selective polishing: the mainte nance of aesthetic restorations. Journal of Practical Hygiene. 2003;12:18-24.
- Barnes CM. Care and maintenance of esthetic restorations. Journal of Practical Hygiene. 2004;14:19-22.
- Covey D, Barnes CM, Watanabe H, Johnson WW. Effects of a paste-free prophylaxis angle and conventional prophylaxis pastes. Gen Dent. 2009. In press.
- Lutz F, Sneer B, Imfeld T, Barbakow F, Schupback P. Self-adjusting abrasiveness: a new technology for prophylaxis pastes. Quintessence Intl. 1993; 24:53-63.
- Litkowski LJ, Hack GD, Sheaffer HB, Greenspan DC. Occlusion of dentinal tubules by 45S5 Bioglass®. In: Bioceramics 10, Proceedings of the 10th International Symposium on Ceramics in Medicine. Sedel L, Rey C, eds. Oxford, England: Aldent Press; 1997:411-414.
From Dimensions of Dental Hygiene. November 2009; 7(11): 18-20, 22.

