Managing Late Implant Complications in a Medically Complex Patient
An 81-year-old patient with well-controlled type 2 diabetes and cardiovascular disease presented with late implant complications, including peri-implant infection and pocketing. A surgical approach combining guided bone regeneration and implant surface detoxification successfully preserved the implant and restored peri-implant health.
An 81-year-old patient with well-controlled type 2 diabetes and cardiovascular disease presented with late implant complications, including peri-implant infection and pocketing. A surgical approach combining guided bone regeneration and implant surface detoxification successfully preserved the implant and restored peri-implant health.
Dental implants offer a reliable solution for edentulous patients, but complications can arise even in well-managed cases. This case study examines the late onset of peri-implant complications in an 81-year-old patient with multiple systemic conditions. Despite a smooth implant placement and initial healing, the patient developed a peri-implant abscess nearly a year after crown delivery. A combination of surgical debridement, guided bone regeneration, and growth factor application was used to manage the infection and promote soft- and hard-tissue healing.
 An 81-year-old man presented to a private practice for implant placement (Figures 1 to 11). He had a medical history of type 2 diabetes mellitus (T2DM), cardiovascular disease, and osteoarthritis. He reported taking metformin, canagliflozin, and amlodipine/benazepril to manage these conditions and an allergy to penicillin. His T2DM was well-controlled with regular monitoring of his blood glucose levels and a self-reported HbA1c of 5.2%.
An 81-year-old man presented to a private practice for implant placement (Figures 1 to 11). He had a medical history of type 2 diabetes mellitus (T2DM), cardiovascular disease, and osteoarthritis. He reported taking metformin, canagliflozin, and amlodipine/benazepril to manage these conditions and an allergy to penicillin. His T2DM was well-controlled with regular monitoring of his blood glucose levels and a self-reported HbA1c of 5.2%.
The patient first presented for extraction and bone grafting of nonrestorable #30 due to a failing root canal. Tooth #30 was extracted in a minimally traumatic fashion. The socket was thoroughly curetted to remove granulation tissue, and an allograft was condensed into the socket. A noncross-linked collagen membrane was prepared and placed under the buccal and the lingual flap margins and stabilized with resorbable sutures.
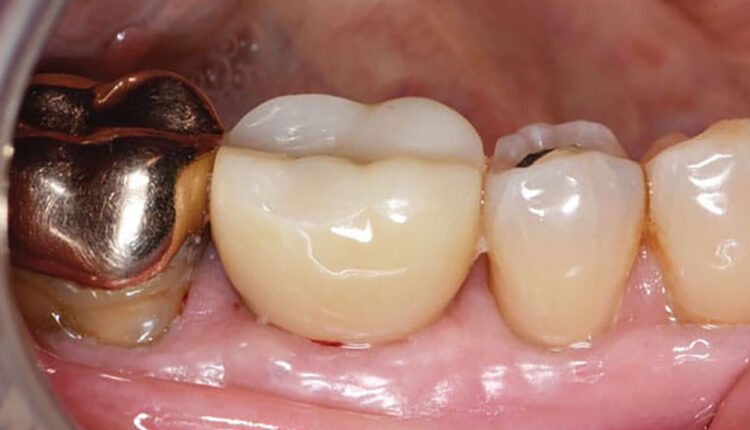
Ridge healing was uneventful. Four months later, after cone-beam computed tomography (CBCT) imaging was obtained, a bone-level, 6 mm-wide and 11.5 mm-long implant was placed at site #30. The patient was referred to his general dentist for implant crown delivery after 5 months of healing.
Nine months after crown insertion, the patient returned for implant evaluation. He complained of swelling and tenderness to percussion and palpation. Clinical examination revealed > 6 mm pocketing between #30/31 with bleeding on probing and suppuration and a draining buccal fistula. Tooth #31 tested normal to vitality testing with no lingering pain nor tenderness to percussion. A size 40 gutta percha master cone was used to trace the fistula to the apical region of the implant. The patient was placed on clindamycin 150 mg and metronidazole 500 mg three times daily for 1 week following American Dental Association guidelines for periapical pain.1
After a thorough evaluation and review of different treatment options, a surgical plan was developed. Due to the radiograph indicating a clearly defined lesion and the use of gutta percha to locate the source of the abscess, an additional CBCT was not deemed necessary. The patient consented to implant flap debridement with guided bone regeneration or implant removal and bone grafting for future implant replacement.
 The patient premedicated with 300 mg of clindamycin 1 hour prior to surgery to minimize complications in early healing.2 Full-thickness flaps were elevated to allow for complete visualization of the intrabony defects. After degranulation with titanium scalers, the two-wall defect measured 9-mm deep between #30/31 and the three-wall defect at #30-M were amendable to grafting. The implant surface was rinsed with 0.12% chlorhexidine and sterile saline. Tetracycline (50 mg/ml) was used to detoxify the implant surface for 2 to 3 minutes followed by another saline rinse. rh-PDGF was then applied to the root and implant surfaces, while a bone allograft was hydrated with this growth factor for approximately 20 minutes before being condensed into the defect. A noncrosslinked collagen membrane was layered over the grafted site. Flaps were repositioned with long-lasting, synthetic, resorbable sutures. The patient was prescribed clindamycin 300 mg and anti-inflammatory and analgesic agents during the perioperative period.
The patient premedicated with 300 mg of clindamycin 1 hour prior to surgery to minimize complications in early healing.2 Full-thickness flaps were elevated to allow for complete visualization of the intrabony defects. After degranulation with titanium scalers, the two-wall defect measured 9-mm deep between #30/31 and the three-wall defect at #30-M were amendable to grafting. The implant surface was rinsed with 0.12% chlorhexidine and sterile saline. Tetracycline (50 mg/ml) was used to detoxify the implant surface for 2 to 3 minutes followed by another saline rinse. rh-PDGF was then applied to the root and implant surfaces, while a bone allograft was hydrated with this growth factor for approximately 20 minutes before being condensed into the defect. A noncrosslinked collagen membrane was layered over the grafted site. Flaps were repositioned with long-lasting, synthetic, resorbable sutures. The patient was prescribed clindamycin 300 mg and anti-inflammatory and analgesic agents during the perioperative period.
After several weeks of healing, the tissues surrounding #29-31 were healthy, pink, and firm. The patient was scheduled for monthly visits to monitor healing and reinforce proper oral hygiene with interproximal aids. Probing depths from #29-31 were < 4 mm at time of the final follow-up. He was referred back to his general dentist and maintained every 4 to 6 months.
This case highlights the importance of long-term monitoring of implant patients, particularly those with systemic conditions that may impact healing. Early intervention and evidence-based surgical management allowed for the successful resolution of peri-implant infection and maintained implant function. Regular follow-ups and patient education on hygiene protocols remain critical for long-term success.
References
- Lockhart P, Tampi M, Abt E, et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J Am Dent Assoc. 2019;150:906-921.
- Arrieta G, Sanchez F, Rodriguez-Audres C, Barbier L, Arteagoitia, I. The effect of preoperative clindamycin in reducing early oral implant failure: a randomized placebo-controlled clinical trial. Cl Oral Invest. 2023;27:1113-1122.
This information originally appeared in Boeriu S, Hottel TL, Saltz AE, et al. A novel approach to treating retrograde peri-implantitis. Decisions in Dentistry. 2024;10(3):36-1.


