Providing the First Line of Defense Against Sleep Breathing Disorders
Dental hygienists play a critical role in identifying these common issues and facilitating early intervention.
This course was published in the March/April 2025 issue and expires April 2028. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 730
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the types of sleep breathing disorders (SBDs).
- Describe the negative health effects of SBDs.
- Discuss the role of dental hygienists in detecting and addressing SBDs.
Dental professionals are at the forefront of screening patients for potential sleep breathing disorders (SBDs) and dental hygienists typically see patients more frequently than other medical providers. As prevention specialists, dental hygienists are able to assess patients and determine their risk for SBDs. Once the risk for an SBD is determined, dental hygienists can refer patients to their primary care provider or sleep specialist.
The most common SBD, obstructive sleep apnea (OSA) is associated with several comorbidities, such as hypertension, depression, chronic fatigue, irregular heartbeat, obesity, and diabetes mellitus.1–3 Sleep is essential for keeping the body and mind balanced and helping the body adjust to internal changes.4,5
Types of Sleep Breathing Disorders
Sleep disorders impact both children and adults but their incidence increases with age.6,7 Sleep disorders may be caused by anatomy, genetics, or medical comorbidities.6
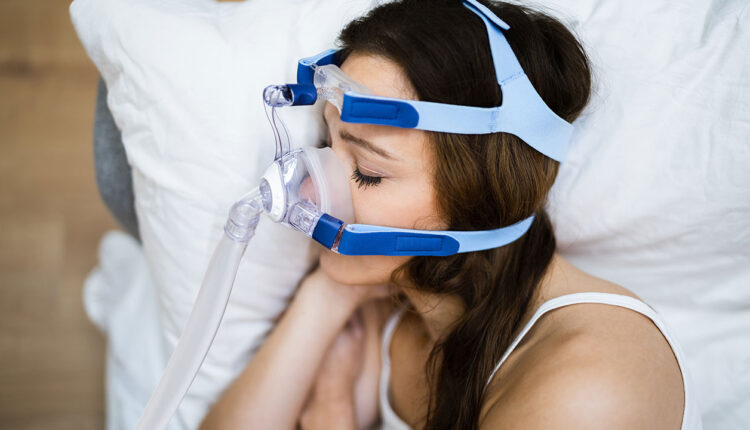
OSA occurs when the airway collapses, leading to temporary pauses in breathing and reduced oxygen levels for 10 seconds or longer. Impacting approximately 30% of American adults, OSA is more common in men than women.6 In the United States, 23 million individuals with OSA remain undiagnosed.6,8,9 Among those diagnosed, 49.7% are men, 23% are women, and 4% are children.6,8,9
Symptoms of OSA are snoring, choking, or gasping during sleep, arousal at nighttime, and daytime sleepiness. In children, signs are enlarged tonsils and adenoids, which can obstruct the airway. Continuous positive airway pressure (CPAP) is the most common treatment.6
Central sleep apnea (CSA) is less common; fewer than 1% of adults older than age 40 are diagnosed. However, CSA is more frequently seen in men older than age 65. CSA occurs when signals from the brain are not appropriately sent to the respiratory system, which prevents the muscles from activating.6 In CSA, disrupted signals from the brain lead to abnormal breathing. While CSA does not involve airway blockage, it can occur alongside OSA, where soft tissue collapse obstructs airflow.6,10
CSA’s signs and symptoms are similar to OSA, including daytime fatigue, frequent awakenings during sleep, and poor concentration.6 While CPAP is often used to treat CSA, it may also contribute to its development. Other treatments include bilevel positive airway pressure (BiPAP) or an adaptive servo-ventilation to back up the respiratory rate with noninvasive airflow.6,10
Complex sleep apnea syndrome (CompSAS) is a manifestation of OSA with CSA components. This respiration pattern can be disruptive or prominent in CompSAS based on the central apnea index. Signs and symptoms are similar to both OSA and CSA; however, the differentiation of CompSAS is based on results from a polysomnography sleep study. CompSAS can be attributed to genetics and environmental factors and occurs mostly among men who are obese.11
Distinctive signs of CompSAS can be identified during a polysomnography sleep study, and specific levels are assesFrtsed during treatment. However, some patients may continue to experience excessive sleepiness even with CPAP therapy.11 The chemoreceptor PaCO2, known as the medullary neuron, plays a role in ventilation and when PaCO2 falls below the threshold, it increases CSA events. Changes in CO2 excretion impact the resistance in the upper airway as well as intracranial hypertension.11–13
Snoring is the sound of the soft palate vibrating during air movement and commonly occurs in men, women, and children. If snoring happens on more than three occasions per week, it can be classified as an SBD. Symptoms contributing to snoring are upper airway discrepancies, such as elasticity of the soft palate, narrow arches, and an enlarged tongue. Other factors that cause snoring include excessive sleepiness, obesity, and the use of alcohol and/or sedatives.6
Upper airway resistance syndrome (UARS) is included within the umbrella of SBDs. UARS is characterized by restricted airflow and increased respiratory effort, leading to sleep disturbances.1 The difference between UARS and OSA is that, in UARS, the oxygen desaturation does not go below 90% despite these disturbances.1,14 A polysomnography sleep study is the best way to diagnose UARS or OSA. Patients with UARS have apneas and hypopneas but fewer incidences of desaturation in oxygen.1
Patients may report daytime fatigue, snoring, morning headaches, depression-like symptoms, sleep disruptions, and frequent episodes of waking. Clinical findings of UARS are the narrowing between the hard palate and uvula, or the uvula and epiglottis. The narrowing impacts the neuromuscular compensatory mechanisms that maintain the airway and increases the resistance in both locations, contributing to frequent respiratory-related arousals. Because there is no substantial drop in oxygen desaturation, UARS tends to go untreated and may progress to OSA.1
Negative Health Effects
SBDs affect the entire body, including the release of hormones. Hormones are critical in controlling vital organs and maintaining balance. The under- or overproducing of hormones negatively impacts the human body’s homeostasis, including igniting an increased inflammatory response. The imbalances lead to comorbidities.2
Endocrine disorders impact SBDs and OSA and vice versa. SBDs are linked to obesity, hypothyroidism, acromegaly, diabetes, Cushing syndrome, and hyperaldosteronism.2,15–18 Individuals who are overweight or obese have a higher prevalence of OSA than those with a healthier body mass index, likely due to fat deposition in the pharyngeal muscles.15–17
Hypothyroidism is an imbalance in hormone levels, creating an underactive thyroid gland. Symptoms are fatigue, weight gain, and bradycardia. Poor sleep hygiene can interact with thyroid functions. Individuals with hypothyroidism are more likely to develop OSA. Thyroid enlargement, known as a goiter, restricts the airway, reducing the movement of air into the lungs.18
Patients with acromegaly —when the body produces too many growth hormones, resulting in larger organs and tissues — have a higher prevalence of OSA.15,19 Patients with acromegaly and OSA tend to have facial skeletal deformities, such as mandible enlargement and thickening of pharyngeal tissue and the tongue.15 Other common oral signs are macroglossia, hypertrophic uvula, and elongated soft palate, which are also frequently found in the upper airways of patients with OSA.20–24
OSA is also highly prevalent in patients with both type 1 and type 2 diabetes. Insulin resistance, autonomic nervous system dysfunction, chronic hyperglycemia, autonomic neuropathy, and microvascular complications are shared by OSA and type 2 diabetes. The International Diabetes Federation recommends that patients with type 2 diabetes get screened for OSA and vice versa.15,25 Patients with type 1 diabetes may have a higher prevalence of OSA due to hyperglycemia.15
Cushing syndrome is characterized by the overproduction of cortisol, the hormone responsible for the stress response. This disorder can also cause insulin resistance, prediabetes, and type 2 diabetes.26 Due to its association with obesity and diabetes, Cushing syndrome is also associated with a higher risk of OSA.15
Aldosterone is a hormone produced by the adrenal glands. Hyperaldosteronism (also known as primary aldosteronism or Conn syndrome) is associated with a type of resistant hypertension.2,27 In patients with OSA, the restriction of aldosterone reduces symptoms. In addition, when there is excess aldosterone, the severity of OSA is greater. Treating hypertension reduces the severity of OSA by 50%.2,28 The use of CPAP in conjunction with medication therapy helps to reduce blood pressure in patients with OSA.2,29
If these endocrine disorders are addressed independently prior to an SBD diagnosis, medications, weight loss, and dietary changes can be implemented. Screening for OSA and these endocrine disorders can prevent the development of other conditions.2,15
Effects on Hormone Release
OSA affects the release of several hormones, such as cortisol and serotonin. Other negative effects are associated with OSA, such as bone formation, sex hormones, hyperaldosteronism, hyperprolactinemia, hypercortisolemia, and inflammatory response. Hormones impact the balance of all systems in the body.2
 Sleep restores neurobehavioral performance and immune function while conserving overall energy, supporting metabolism, and replenishing brain energy. Sleep impacts the balance of the hypothalamus-pituitary-adrenal (HPA) axis. The awakenings in patients with OSA impair the activity of the HPA axis, leading to heightened levels of cortisol release and autonomic activation. Due to the chronic disruption of the circadian rhythm, the association with the master hypothalamic clock and pacemakers in peripheral tissues creates a nonsynchronous rhythm.2,30
Sleep restores neurobehavioral performance and immune function while conserving overall energy, supporting metabolism, and replenishing brain energy. Sleep impacts the balance of the hypothalamus-pituitary-adrenal (HPA) axis. The awakenings in patients with OSA impair the activity of the HPA axis, leading to heightened levels of cortisol release and autonomic activation. Due to the chronic disruption of the circadian rhythm, the association with the master hypothalamic clock and pacemakers in peripheral tissues creates a nonsynchronous rhythm.2,30
The inflammatory response is increased due to oxidative stress from fatigue, lack of sleep, and oxygen deprivation, increasing inflammation and markers for heart disease and hypertension. SBDs must be treated to reduce the release of cortisol, pro-inflammatory cytokines, interleukin-6, and tumor necrosis factor. Inadequate release of vitamin D is also caused by SBDs and may lead to osteopenia or osteoporosis.2
Sex hormones and breathing influence each other simultaneously. SBDs and OSA negatively impact hormonal homeostasis in both men and women.2 Testosterone production is affected by sleep disturbances because it peaks in the blood during rapid eye movement sleep and reaches its lowest in the afternoon.31 Sleep deprivation as well as the side effects of OSA lowers testosterone levels in men.2
Polycystic ovarian syndrome is a common endocrine disorder in women, primarily among premenopausal women who are obese. The lack of hormonal homeostasis in women affects progesterone and estradiol levels, and women with polycystic ovarian syndrome experience high amounts of sleep disturbances. These long-term ramifications lead to insulin resistance, metabolic syndrome, heart disease, and mental health disorders. Sleep disorders may be linked to miscarriages and polycystic ovarian syndrome.2
Hyperprolactinemia is an increased level of prolactin (PRL) in the blood. This hormone contributes to hypothyroidism and tumors around the pituitary gland. The secretion of PRL is related to hypoxic stress and the rhythm of PRL release. However, significant changes are possible with CPAP therapy.2,32
Role of Dental Hygienists
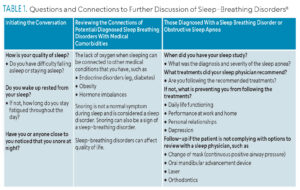
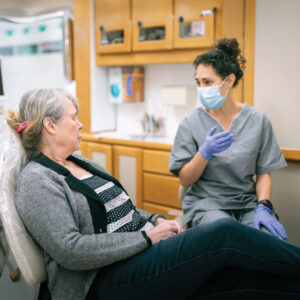
Dental hygienists play a critical role in bridging the gap between oral and whole-body health. Clinical findings and discussions on patients’ symptoms related to their oral cavity and upper airway allow oral healthcare providers to identify a potential upper airway discrepancy. Dental hygienists can initiate the conversation (Table 1).
Discussions on SBDs and OSAs should be included during patient assessments and evaluations. Dental hygienists can refer patients back to their sleep physicians if issues are detected. This allows interprofessional collaboration between dental providers and sleep specialists.
![]() Conclusion
Conclusion
Dental hygienists provide screenings that can save their patients’ lives. Screening for sleep apnea is critical because of the link between the oral cavity and the upper respiratory tract. SBDs, including OSA, are correlated with endocrine disorders and hormone release. Therefore, dental hygienists can utilize a comprehensive medical history, intraoral examination, and ask the appropriate questions to connect these conditions. Based on these findings, referrals to sleep physicians can be determined, enhancing interprofessional collaboration.
References
- Maggard M, Sankari A, Cascella M. Upper Airway Resistance Syndrome. Treasure Island, Florida: StatPearls Publishing; 2025.
- Ruchała M, Bromińska B, Cyrańska-Chyrek E, Kuźnar-Kamińska B, Kostrzewska M, Batura-Gabryel H. Obstructive sleep apnea and hormones — a novel insight. Arch Med Sci. 2017;13:875–884.
- Knauert M, Naik S, Gillespie M, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. WorlJ J Otorhinolaryngol Head Neck Surg. 2015;1:17–27.
- Garbarino S, Lanteri P, Durando P, Magnavita N, Sannita W. Co-morbidity, mortality, quality of life and the healthca/e/welfare/social costs of disordered sleep: A rapid review. Int J Environ Res Public Health. 2016;13:831.
- Kornegay E, Brame J. Obstructive sleep apnea and the role of dental hygienists. J Dent Hyg. 2015;89:286–292.
- Suni E, Singh A. Sleep-Related Breathing Disordersj. Available at sleepfoundation.org/sleep-related-breathing-disorders. Accessed February 11, 2025.
- Sadaf M, Johnson A, Daw J, Hashmi A, Khawaja I. Breathing-related sleep disorders in the elderly. Psychiatr Ann. 2018;48:287–291.
- Cuzella V, Dean MC. The role of dental hygienists in addressing sleep breathing disorders. Dimensions of Dental Hygiene. 2020;18(11):32–35.
- Gianoni-Capenakas S, Gomes A, Mayoral P, Miguez M, Pliska B, Lagravere M. Sleep-disordered breathing: the dentists’ role – a systematic review. J Dent Sleep Med. 2020;7:1–15.
- Sigua N, Kakazu M. What is central sleep apnea in adults? Am J Respir Crit Care Med. 2021;203:P18–19.
- Wang J, Wang Y, Feng J, Chen BY, Cao J. Complex sleep apnea syndrome. Patient Prefer Adherence. 2013;7:633–641.
- Skatrud J, Dempsey J, Badr S, Begle R. Effect of airway impedance on CO2 retention and respiratory muscle activity during NREM sleep. J Appl Physiol. 1988;65:1676–1685.
- Poca M, Ferre A, de la Calzada M, Moncho D, Fernandez-Torrelles S, Sahuquillo J. CO2-induced intracranial hypertension and high-amplitude B-waves in a patient with Chiari 1 malformation and sleep apnea syndrome that resolved following CPAP therapy. Acta Neurochir. 2021;163:3075–3082.
- Fabius T, Benistant J, Bekkedam L, van der Palen J, de Jongh F, Eijsvogel M. Validation of the oxygen desaturation index in the diagnostic workup of obstructive sleep apnea. Sleep Breath. 2019;23:57–63.
- Akset M, Poppe K, Kleynen P, Bold I, Bruyneel M. Endocrine disorders in obstructive sleep apnoea syndrome: A bidirectional relationship. Clin Endocrinol. 2023;98:3–13.
- Gottlieb D, Punjabi N. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA. 2020;323:1389–1400.
- Kim A, Keenan B, Jackson N, Chan E, Staley B, Poptani H, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37:1639–1648.
- Thavaraputta S, Dennis J, Laoveeravat P, Nugent K, Rivas A. Hypothyroidism and its association with sleep apnea among adults in the United States: NHANES 2007–2008. J Clin Endocrinol Metab. 2019;104:4990–4997.
- National Institute of Diabetes and Digestive and Kidney Diseases. Acromegaly. Available at: niddk.nih.gov/health-information/endocrine-diseases/acromegaly. Accessed February 10, 2025.
- Vilar L, Vilar C, Lyra R, Lyra R, Naves L. Acromegaly: clinical features at diagnosis. Pituitary. 2017;20:22–32.
- Kreitschmann-Andermahr I, Kohlmann J, Kleist B, Hirschfelder U, Buslei R, Buchfelder M, et al. Oro-dental pathologies in acromegaly. Endocr. 2018;60:323–328.
- Roumeau S, Thevenon J, Ouchchane L, Maqdasy S, Batisse-Lignier M, Duale C, et al. Assessment of oro-dental manifestations in a series of acromegalic patients, the AcroDent study. Endocr Connect. 2020;9:824–833.
- Abreu A, Tovar A, Castellanos R, Valenzuela A, Giraldo C, Pinedo A, et al. Challenges in the diagnosis and management of acromegaly: a focus on comorbidities. Pituitary. 2016;19:448–457.
- Dostalova S, Sonka K, Smahel Z, Weiss V, Marek J. Cephalometric assessment of cranial abnormalities in patients with acromegaly. J Craniomaxillofac. 2003;31:80–87.
- International Diabetes Federation. The IDF Consensus Statement on Sleep Apnoea and Type 2 Diabetes. Available at h/tps://idf.org/media/uploads/떗/葑/attachments-32.pdf. Accessed February 10, 2025.
- National Institute of Diabetes and Digestive and Kidney Diseases. Cushing’s Syndrome. Available at niddk.nih./ov/health-information/endocrine-diseases/cushings-syndrome#:~:text=Cushing’s%20syndrome%20is%20a%20disorder,glucose%2C%20also%20called%20blood%20sugar. Accessed February 10, 2025.
- Grossman A. Primary aldosteronism. Available at merckmanuals.com/professional/endocrine-and-metabolic-disorders/adrenal-disorders/primary-aldosteronism. Accessed February 10, 2025.
- Calhoun D, Nishizaka M, Zaman M, Harding S. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125:112–117.
- Schein A, Kerkhoff A, Coronel C, Plentz R, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 paJ ents. J Hypertens. 2014;32:1762–1773.
- Ekstedt M, Akerstedt T, Söderström M. Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure. Psychosom Med. 2004;66:925–931.
- Liu P, Reddy R. Sleep, testosterone and cortisol balance, and ageing men. Rev Endocr Metab Disord. 2022;23:1323–1339.
- Johns Hopkins Medicine. Hyperprolactinemia. Available at hopkinsmedicine.org/health/conditions-and-diseases/hyperprolactinemia#:~:text=What%20is%20hyperprolactinemia%3F,controls%20production%20of%20this%20hormone. Accessed February 10, 2025.
From Dimensions of Dental Hygiene. March/April 2025; 23(2):42-45.