 VAGENGEYM_ELENA/ISTOCK/GETTY IMAGES PLUS
VAGENGEYM_ELENA/ISTOCK/GETTY IMAGES PLUS
Advancing In-Office Whitening Techniques
As patients continue to demand whiter teeth, new in-office bleaching techniques help make positive outcomes easier than ever.
This course was published in the October 2019 issue and expires October 2022. Sibel A. Antonson, DDS, PhD, MBA, discloses an honorarium from Colgate-Palmolive Co. Ahmed J. Abuzinadah, DDS, has nothing to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify early clinical advocates for dental whitening procedures, and patient perceptions that drive demand for bleaching.
- Explain possible etiologies for discolored teeth, the main mechanism of action for dental bleaching, and a common side effect of whitening therapy.
- Describe various techniques and agents used for tooth bleaching.
INTRODUCTION
As dental professionals, we are well aware that tooth whitening is one of the most common requests when patients want to improve their smile. Whitening methods have undergone significant transformation and innovation in recent years, so it is important for clinicians to keep up with the latest techniques. Colgate is proud to support the development of this continuing education article, which provides an overview of whitening options—including toothpastes, home bleaching, and in-office whitening treatment.
The authors explain the merits of each approach, and present two case reports using Colgate’s new Optic White Professional bleaching system. This innovative treatment provides fast and effective whitening results, and offers clinicians and patients the choice of in-office or home treatment. In-office whitening requires just a single appointment, and Colgate’s advanced ionic technology and adjustable mouthpiece eliminate the need for light, heat, or custom trays. Designed to avoid dentinal sensitivity, this state-of-the-art system will transform your patients’ whitening experience.
I hope you will find the article a valuable resource and embrace the key points of this new advanced ionic technology.
—Matilde Hernández, DDS, MS, MBA
Scientific Affairs Manager Professional Oral Care
Colgate Oral Pharmaceuticals
Professional tooth whitening, including new approaches to office bleaching procedures, can help patients achieve whiter and brighter smiles
The desire for a confident smile is perhaps one of the earliest human attempts to improve our appearance as a social species. Dental bleaching was first documented when a technique for bleaching was described by Fitch1 in 1861, nearly 160 years ago. According to a survey by the American Academy of Cosmetic Dentistry, 96% of respondents agreed that an attractive smile makes them more appealing to the opposite sex. Most participants also stated that having whiter and brighter teeth would improve their smile. This is no surprise as smiles are one of the most powerful communication and engagement vehicles humans have. In fact, the inability to smile can become a social disability, causing many inconveniences in social interactions. The survey supports this notion, as 74% of the participants believed that an unattractive smile hurts their chances of career success.2 This interest in whiter teeth led to an 1882 paper by Harlan3 proposing an in-office bleaching protocol. In 1937, more than 50 years later, Ames4 reported the use of hydrogen peroxide as a bleaching agent.
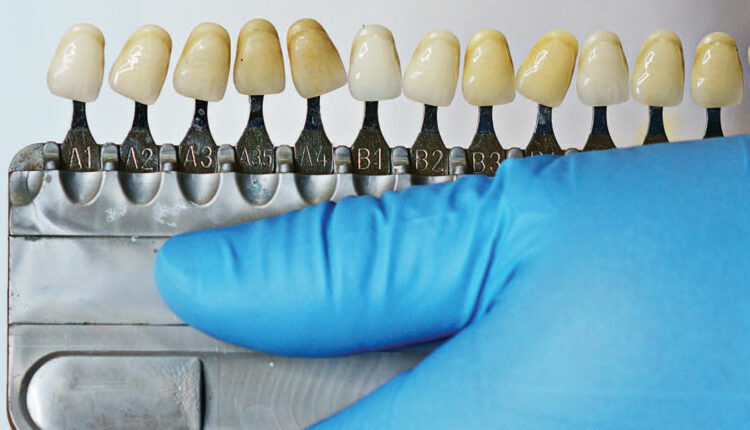
Commercially available dental bleaching procedures became popular in 1989 with the introduction of a stable and safe delivery mechanism of the active bleaching agents. The use of carbamide peroxide in a gel with vacuum-formed custom trays as carriers allowed the controlled placement of the bleaching agent on targeted teeth for the desired time. This technique, referred to as night guard vital bleaching, provided a safe and effective method for tooth whitening.5
Compared with achieving the same outcome via restorative procedures, bleaching is a minimally invasive approach to helping patients address esthetic concerns safely and in relatively rapid fashion. Prior to the commercial availability of dental bleaching systems, clinicians relied on direct and indirect restorative techniques to provide patients with brighter smiles.6
Tooth bleaching is not only a minimally invasive approach, it is also the most economic esthetic solution for patients. A positive outcome often increases a patient’s motivation and awareness of other dental concerns, which become evident as a result of the whitening treatment. Often, patients are more motivated to take an active role in self-care after bleaching therapy. They are more engaged and, thus, more likely to accept proposed treatment plans.
ETIOLOGY OF DISCOLORED TEETH
In clinical practice, providers need to diagnose the etiology of discolored teeth and provide a suitable treatment plan, as well as related patient education. Discoloration is due to either intrinsic or extrinsic factors. The latter might include deposits or diffusion of chromogenic foods or agents, such as tobacco, coffee, red wine, beets, or turmeric. These components are mainly superficial on the enamel level, and are more predictably treated with bleaching procedures. Other whitening approaches, such as whitening dentifrices, can provide a favorable outcome for extrinsic stains, as well. Intrinsic causes stem from hereditary or other factors, such as darker dentin color, genetic disorders, densified dentin due to aging, infectious diseases, trauma, and medications, such as tetracycline.7–11 Intrinsic discoloration is more resistant to bleaching procedures, and successful outcomes should be expected to take longer. This is why patient education is helpful for managing expectations prior to the start of treatment.
BLEACHING MECHANISM OF ACTION
The main mechanism of action for dental bleaching is an oxidative process; it is believed this creates free radicals of oxygen that break down large chromogenic molecules into smaller molecules. The resulting molecules are less perceptible due to their inability to reflect light. In addition, their size also allows for their easy removal from the dentin/enamel complex.12 Hydrogen peroxide is one of the chief agents used for dental bleaching, and it is highly diffusive. This diffusivity allows it to penetrate enamel and reach dentin. Carbamide peroxide ranks as another commonly used ingredient. This agent is a stable form of hydrogen peroxide that breaks down to produce hydrogen peroxide that is approximately one third of its original volume; for example, 30% carbamide peroxide produces 10% hydrogen peroxide.12 Sodium perborate and chlorine dioxide have also been used for bleaching procedures. However, chlorine dioxide is not considered a safe option for dentistry. Sodium perborate is available in a powder form, and is mixed with distilled water for activation. In mixed form, it produces a concentration of 16% hydrogen peroxide.
Due to the processes of diffusion and oxidation, achieving faster results with extrinsic staining is expected, while some intrinsic staining, such as sclerotic dentin and tetracycline, takes longer to achieve the desired result. A higher concentration of hydrogen peroxide can provide faster results—although the occurrence of dentinal hypersensitivity and safety of the soft tissues become a concern.13–15
There are many approaches to dental bleaching. While a successful outcome can be expected with either professionally delivered or over-the-counter whitening strategies, this is mainly dictated by the staining’s etiology. Simply put, procedural efficacy depends on the concentration of the agent and time it is applied: The higher the concentration, the faster the response, and vice versa.12–15 Therefore, the lower-concentration, over-the-counter products provide a superficial level of bleaching that is mainly effective for mild extrinsic staining. By comparison, professional office bleaching procedures can effectively treat intrinsic staining, as well as moderate to severe extrinsic staining.
Professional procedures offer the clinician many strategies, based on either delivery, concentration, or activation methodologies. All rely on breaking down the hydrogen peroxide molecules to create oxygen molecules as free radicals. In general, providers have three whitening options to offer, and can also suggest a combination approach.
Over-the-counter whitening products: These contain the lowest concentration of active whitening ingredients and their effectiveness is limited to extrinsic discoloration. This category includes dentifrices containing abrasive particles (similar to coarse prophy pastes) to remove deposits of chromogenic agents. However, their abrasive properties also increase the surface roughness of the enamel, causing higher retention rates of chromogenic agents.16–18
Professionally supervised at-home bleaching: Patients administer the bleaching procedure at home using custom trays and bleaching agents supplied by the dentist. The objective of the custom tray is to retain the gel on the tooth surface for an extended period, while avoiding gel contact with the soft tissues. Typically, a successful outcome can be achieved in 2 weeks.19
In-office bleaching: Higher-concentration bleaching agents are applied in the office, and these concentrations require clinicians to protect the gingiva with a resin-based material to avoid irritation. These bleaching systems often utilize heat to accelerate the breakdown of hydrogen peroxide. In many cases, heat is provided with a light source. Dentinal hypersensitivity is a main concern with these procedures.20
Combination treatment: A dual approach involving at-home and office bleaching can be a viable option for patients with intrinsic staining who would like to achieve faster results—and with a more stable outcome.21 This management technique is typically applied either due to patients’ demand for faster outcomes, or their inability or unwillingness to wear the trays at home for long periods. Research has shown the combination of in-office treatment and 1-week at-home treatment provides a faster and more stable outcome than other approaches. However, after 2 weeks, there was no difference between the combination treatment and professional at-home bleaching alone.21 This outcome validates the effectiveness of professional at-home bleaching treatment, as long as it is used consistently and according to the manufacturers’ instructions for 2 weeks.
OFFICE BLEACHING SYSTEMS
While clinicians can choose from several methods to activate dental bleaching agents, such as the application of heat and light, there are conflicting reviews about the effectiveness of these external activation sources. A 10° C increase in temperature increases the speed of hydrogen peroxide breakdown by 2.2 times. In order to facilitate this acceleration, a heat source is applied to the bleaching agent to hypothetically accelerate the breakdown process, thus increasing its effectiveness in a shorter period. These heat sources are often high-powered lights or lasers. However, this temperature increase can potentially damage the pulp.22 Many of the lights marketed for dental bleaching procedures are mainly heat sources. Yet despite the market popularity of these lights, studies suggest their effectiveness is questionable.21,23,24
Additionally, photolysis of hydrogen peroxide is possible by applying ultraviolet spectrum lights—although this method is not recommended due to safety concerns.22 On the other hand, violet light-emitting diode application, along with bleaching gels, have recently shown promise.25–27
Of note, the majority of these light-activated heat systems cause dehydration due to heat, and, as a result, teeth appear lighter immediately after the procedure. In many cases, however, the combination of heat and dehydration causes sensitivity during office bleaching procedures.
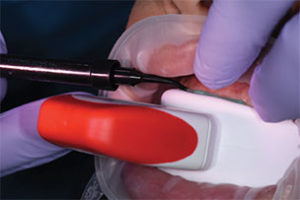
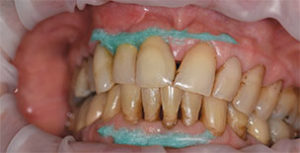
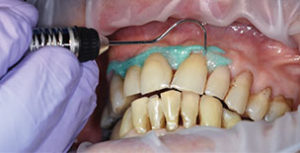
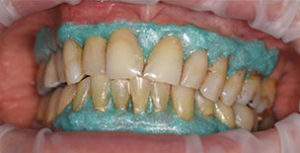
High concentration of the active agents used for office bleaching can cause severe gingival irritation. Therefore, gingival barriers are an essential component in these procedures. Gingival barriers are light-curable resin materials applied and polymerized over the gingiva and up to the gingival margin on the cervical area of the teeth. The operator must ensure effective sealing of the facial gingiva, and if there are diastemas, the lingual cervical area must also be covered with the gingival barrier to avoid potential irritation (Figure 1 through Figure 4). In case of an inadvertent irritation, the area should be rinsed copiously with water. Typically, the gingival irritation regresses within 24 hours to 48 hours.27
MANAGING SENSITIVITY
As noted, sensitivity is a main concern with office bleaching therapy and it affects approximately 70% of patients.21,28–31 Even though sensitivity is temporary, patient discomfort may be a clinical deterrent in administering bleaching systems.32,33 Application of fluoride before and after whitening treatment may help relieve symptoms; however, complete elimination of sensitivity with the various office bleaching methods has not been possible. In addition to fluoride application, incorporation of potassium nitrate and calcium has been shown to be an effective method of reducing sensitivity.30,33
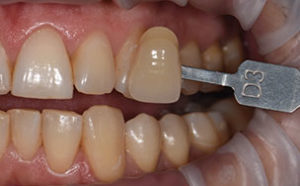
NEW APPROACH TO BLEACHING
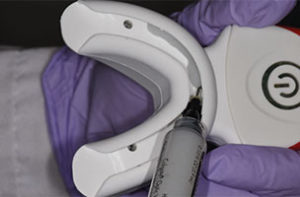
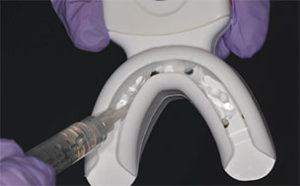
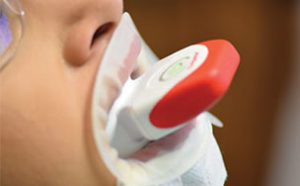
There is new approach to bleaching that utilizes a unique and proprietary ionic technology—without the presence of light or heat—to accelerate the breakdown of hydrogen peroxide. Clinicians should note that applying a gingival barrier is also necessary in this procedure to protect the soft tissues. With this method, a specifically formulated hydrogen peroxide gel is delivered into a proprietary tray that contains a battery in a sealed system. The gel is used on the facial surface, along with a secondary conducting gel covering the incisal and lingual surfaces. When the system is initiated, electrochemical action activates the ionization process. The resulting ionization aids in the breakdown of the hydrogen peroxide and also raises the mixture’s pH. The manufacturer recommends three 10-minute application cycles. The gel and teeth remain hydrated throughout the procedure, and patients do not experience any sensation of the electrochemical process. This method is also safe to use on individuals with cardiac pacemakers.
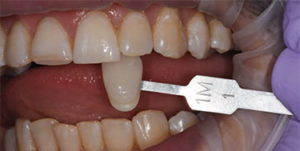
The trays are designed to administer the bleaching agent to the upper and lower arches at the same time. Patients with heavy and intrinsic staining can benefit from a combination treatment approach, and the same tray can be used for the at-home-bleaching step using a proprietary 9% hydrogen peroxide agent.
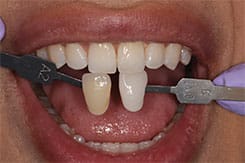
Because custom trays are not needed, this new system saves chairtime, as well as costs associated with making impressions, and fabricating models and custom trays. It is also comfortable for patients as they can remain occluded during the procedure. Figure 5 through Figure 7 demonstrate the clinical application of this technique.
CASE REPORTS
Results from this new ionic whitening system are seen in the figures accompanying these brief case reports.
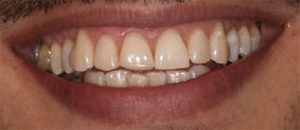
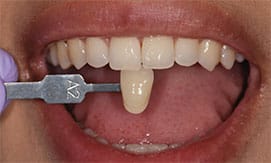
Patient A presented with multiple existing defective restorations and systemically discolored teeth. A bleaching procedure was recommended prior to replacing his existing restorations, and also to ensure minimally invasive restorative treatment with the help of a desirable initial shade. The outcome proved successful and the patient reported no sensitivity as a result of treatment (Figure 8 through Figure 10).
Patient B, who is a heavy coffee and tea drinker, presented with extremely sensitive dentition, to the point where she was unable to use even the low-concentration, at-home bleaching agents in the past. A full-cycle ionic procedure was completed without sensitivity issues, and the outcome was deemed successful (as seen in Figure 11 and Figure 12).
CONCLUSION
All dental team members can incorporate office bleaching discussions into their patient interactions. The best way to articulate the value of dental bleaching is by helping the patient envision a successful outcome in a bleached and well-maintained dentition.
This presentation fosters further discussion regarding overall patient outcomes and experiences. It also promotes communication between providers and patients regarding appropriate and patient-specific clinical options for whitening, expectations, and the anticipated timing and outcome.
REFERENCES
- Fitch C. Etiology of the discoloration of teeth. Dent Cosmos 1861;3:133–136.
- American Academy of Cosmetic Dentistry. State of the Cosmetic Dentistry Industry 2017 Survey Report. Available at: aacd.com/2017survey. Accessed September 24, 2019.
- Harlan AW. Hydrogen dioxide (in the treatment of alveolar abscess, pyorrhea and the bleaching of teeth). Dent Cosmos. 1882;24:515–523.
- Ames J. Removing stains from mottled enamel. J Am Dent Assoc. 1937;24:1674–1677.
- Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence Int. 1992;23:471–488.
- Haywood VB, Al Farawati F. Bleaching update and the future impact on prosthodontics. Br Dent J. 2019;226:753–760.
- Joiner AJ, Raven NM, Jones SJ. Investigation of factors influencing stain formation utilizing an in situ model. Adv Dent Res. 1995;9:471–476.
- ten Bosch JJ, Coops JC. Tooth color and reflectance as related to tooth scattering and enamel hardness. J Dent Res. 1995;74:373–380.
- Goldstein RG, Garber DA. Complete Dental Bleaching. Chicago, Ill: Quintessence Publishing; 1995:73–74.
- Dayan DH, Heifferman A, Gorski M, Begleiter A. Tooth discoloration — extrinsic and intrinsic factors Quintessence Int Dent Dig. 1983;14:195–199.
- Hayes PF, Full C, Pinkham C. The aetiology and treatment of intrinsic discolourations. J Can Dent Assoc. 1986;52:217–220.
- Kwon SR, Wertz PW. Review of the mechanism of tooth whitening. J Esthet Restor Dent. 2015;27:240–257.
- Bersezio C, Estay J, Jorquera G, et al. Effectiveness of dental bleaching with 37.5% and 6% hydrogen peroxide and its effect on quality of life. Oper Den. 2019;44:146–155.
- L Darriba I, Cabirta Melón P, García Sartal A, Ríos Sousa I, Alonso de la Peña V. Influence of treatment duration on the efficacy of at-home bleaching with daytime application: a randomized clinical trial. Clin Oral Investig. 2019;23:3229–3237.
- Ferraz NK, Nogueir LC, Neiva IM, Ferreira RC, Moreira AN, Magalhães CS. Longevity, effectiveness, safety, and impact on quality of life of low-concentration hydrogen peroxides in-office bleaching: a randomized clinical trial. Clin Oral Investig. 2019;23:2061–2070.
- Antonson SA, Yazici AR, Kilinc E, Antonson DE, Hardigan PC. Comparison of different finishing/polishing systems on surface roughness and gloss of resin composites. J Dent. 2011;39(Suppl 1):e9–e17.
- Alsharari T, Garashi M, Hardigan P, Antonson S. Effect of prophylactic pastes on surface gloss of ceramics. J Dent Res. 2018;97:105.
- Alsharari T, Garashi M, Hardigan P, Antonson S. Effect of prophylactic pastes on surface roughness of resin composites. J Dent Res. 2018;97:106.
- Haywood VB. Nightguard vital bleaching: current concepts and research. J Am Dent Assoc.1997;128(Suppl):19S–25S.
- Greenwall L. Bleaching Techniques in Restorative Dentistry. London: Martin Dunitz; 2002.
- Bernardon JK, Sartori N, Ballarin A, Perdigão J, Lopes GC, Baratieri LN. Clinical performance of vital bleaching techniques. Oper Dent. 2010;35:3–10.
- Buchallaa W, Attin A. External bleaching therapy with activation by heat, light or laser — a systematic review. Dent Mater. 2007;23:586–596.
- Maran BM, Ziegelmann PK, Burey A, de Paris Matos T, Loguercio AD, Reis A. Different light-activation systems associated with dental bleaching: a systematic review and a network meta-analysis. Clin Oral Investig. 2019;23:1499–1512.
- SoutoMaior JR, de Moraes S, Lemos C, Vasconcelos BD, Montes M, Pellizzer EP. Effectiveness of light sources on in-office dental bleaching: a systematic review and meta-analyses. Oper Dent. 2019;44:E105–E117.
- de Almeida EN, Besegato JF, Dos Santos DD, de Souza Rastelli AN, Bagnato VS. Violet LED for non-vital tooth bleaching as a new approach. Photodiagnosis Photodyn Ther. Aug 21, 2019. Epub ahead of print.
- Brugnera AP, Nammour S, Rodrigues JA, et al. Clinical evaluation of in-office dental bleaching using a violet LED. Photobiomodul Photomed Laser Surg. Aug 22, 2019. Epub ahead of print.
- Fiorillo L, Laino L, De Stefano R, et al. Dental whitening gels: strengths and weaknesses of an increasingly used method. Gels. 2019;5:E35.
- Marson FC, Sensi LG, Vieira LC, Araújo E. Clinical evaluation of in-office dental bleaching treatments with and without the use of light-activation sources. Oper Dent. 2008;33:15–22.
- de Paula EA, Nava JA, Rosso C, et al. In-office bleaching with a two- and seven-day intervals between clinical sessions: a randomized clinical trial on tooth sensitivity. J Dent. 2015;43:424–429.
- Kossatz S, Martins G, Loguercio AD, Reis A. Tooth sensitivity and bleaching effectiveness associated with use of a calcium-containing in-office bleaching gel. J Am Dent Assoc. 2012;143:e81–e87.
- Martin J, Fernandez E, Bahamondes V. Dentin hypersensitivity after teeth bleaching with in-office systems. Randomized clinical trial. Am J Dent. 2013;26:10–14.
- Kossatz S, Dalanhol AP, Cunha T, Loguercio A, Reis A. Effect of light activation on tooth sensitivity after in-office bleaching. Oper Dent. 2011;36:251–257.
- Haywood VB. Treating sensitivity during tooth whitening. Compend Contin Educ Dent. 2005;26(9 Suppl 3):11–20.
From Dimensions of Dental Hygiene. October 2019;17(9):25—30.



