
Balancing Act
Carlos González-Cabezas, DDS, MSD, PhD, discusses the ongoing remineralization/demineralization process that occurs in the oral cavity and its effect on oral health.
Oral health is based on a delicate balancing act. When the remineralization/ demineralization process vacillates between losing and gaining minerals—with neither taking the lead—health is maintained. But when mineral loss occurs more often than mineral gain for a significant amount of time, dental caries is the result. Dr. González-Cabezas explains how this process works and what can be done to maintain the right balance.
How does the remineralization/demineralization process work in the mouth?
The tooth surface regularly goes through mineral gain (remineralization) and mineral loss (demineralization)—particularly when it is covered by dental biofilms (ie, dental plaque). These biofilms support the exchange of minerals by creating conditions of unsaturation and supersaturation. Unsaturation happens during acidic conditions in the biofilm when the number of available minerals is reduced. This is a common occurrence in the presence of fermentable carbohydrates.1 Supersaturation occurs after the pH is raised by different buffering systems, reduced availability of fermentable carbohydrates, and the increase of minerals from saliva, bacteria, calculus, calcium-fluoride formulations, and the tooth surface.2 This increase in saturation level in the biofilm stops the mineral loss of the enamel and allows some of the minerals to return to the partially demineralized enamel crystals. This happens several times a day, particularly after the consumption of carbohydrates. The remineralization/demineralization process is a normal physiological response and, in effect, enhances the quality of the enamel on the tooth surface.
What role does this process play in the development of caries?
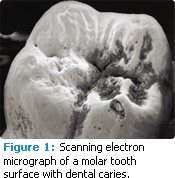 This process is essential in the development of carious lesions. The lesions develop in the enamel or dentin surface when more mineral is lost than is gained over long periods during this remineralization/demineralization process. At the early stages of enamel demineralization, the tooth surface then becomes more rough and porous (Figure 1).
This process is essential in the development of carious lesions. The lesions develop in the enamel or dentin surface when more mineral is lost than is gained over long periods during this remineralization/demineralization process. At the early stages of enamel demineralization, the tooth surface then becomes more rough and porous (Figure 1).
Can carious lesions be arrested?
Yes, any lesion can be arrested, even a cavitated one. When the scale first tips too heavily toward demineralization, only small changes of the tooth structure occur and they can probably be easily remineralized. The problem is that these very early noncavitated lesions are not detectable by traditional methods—eg, visual, tactile, or radiographically—making them difficult to address early when the process is easier to reverse. If a noncavitated lesion is not remineralized, the disease process continues until the structure of the lesion is unable to withstand everyday stresses, such as toothbrushing and chewing, and the surface collapses.2 Once complete cavitation has occurred, restorative care is almost always needed because the tooth morphology tends to trap biofilm, creating a very cariogenic environment. On the other hand, subsurface lesions that are incipient can be reversed if they are treated before complete cavitation takes place.
How can incipient (noncavitated) lesions be reversed?
One way is to decrease the amount of biofilm in the area. This reduces the pH, decreasing the frequency and the intensity of the unsaturation periods. Another strategy is to reduce the frequency of carbohydrate ingestion. This limits the amount of acid being produced in the biofilm, which allows more time for supersaturation to occur. An additional approach is to increase the amount of and concentration of fluoride for those at high risk. Fluoride greatly impacts the saturation levels—decreasing mineral loss and increasing mineral gain. This is why fluoride is the most important tool in the caries prevention armamentarium.
Are there any other tools that can be used to help boost the remineralization process?
Calcium phosphate technologies are a relatively new strategy used to increase mineral gain. Ongoing in vivo research is needed to confirm their efficacy but they can be positive adjuncts to fluoride use. They work by increasing the concentration of available calcium and phosphate ions at the tooth surface. The different technologies, which include amorphous calcium phosphate (ACP), casein phosphopeptide amorphous calcium phosphate (CPP-ACP or Recaldent®), calcium sodium phosphosilicate (CSP or NovaMin®), and tri-calcium phosphate (TCP), have different ways of accomplishing this. ACP delivers calcium salt and phosphate salt separately (eg, via a syringe), to create a reservoir of calcium and phosphate ions to fill defects on the tooth surface.3 Recaldent uses a protein from milk that holds calcium and phosphate, and attaches to biofilm.
When the pH in the biofilm drops, the protein releases calcium and phosphate, which then increase the saturation of those minerals in the area.4 Novamin is a bioactive glass that releases ions of calcium, phosphate, and sodium when exposed to saliva.5 TCP is a combination of beta tricalcium phosphate and sodium lauryl sulfate, which creates a “functionalized” calcium and a “free” phosphate, that are designed to improve fluoride remineralization.6 Calcium phosphate technologies are typically recommended for patients at high risk of caries as a supplement to fluoride usage.
Are there any other new technologies on the horizon to improve the likelihood of remineralization in the oral environment?
Many different ideas are being studied but so far none have provided the same results as fluoride—the gold standard. I think a more significant change will come from a better understanding of caries as a disease process rather than treating the condition. Currently, the focus has shifted to identifying those patients who are at greatest risk of developing caries, and employing prevention therapies as early as possible.
The views expressed in this interview are those of Carlos González-Cabezas, DDS, MSD, PhD.
PHOTO CREDIT: FIGURE 1: DAVID MCCARTHY/SCIENCE PHOTO LIBRARY
REFERENCES
- Margolis HC, Zhang YP, van Houte J, et al. Effect of sucrose concentration on the cariogenic potential of pooled plaque fluid from caries-free and caries-positive individuals. Caries Res. 1993;27:467-473.
- González-Cabezas C. The chemistry of caries: remineralization and demineralization events with direct clinical relevance. Dent Clin N Am. 2010;54:469-478.
- Tung MS, Eichmiller FC. Amorphous calcium phosphates for tooth remineralization. Compend Contin Educ Dent. 2004;25(Suppl):9-13.
- Srinivasan N, Kavitha M, Loganathan SC. Comparison of the remineralization potential of CPP-ACP and CPP-ACP with 900 ppm fluoride on eroded human enamel: An in situ study. Arch Oral Biol. 2010;55:541-544.
- Andersson OH, Kangasniemi I. Calcium phosphate formation at the surface of bioactive glass in vitro. J Biomed Mater Res. 1991;25:1019-1030.
- Karlinsey RL, Mackey AC. Solid-state preparation and dental application of an organically modified calcium phosphate. J Mater Sci. 2009;44:346-349.
From Dimensions of Dental Hygiene. March 2012; 10(3): 46, 48.

