Study Takes a Fresh Approach to Plaque Disruption in Early Childhood Caries
Scientists strategize to disrupt stubborn bacteria-yeast biofilm implicated in early childhood caries.

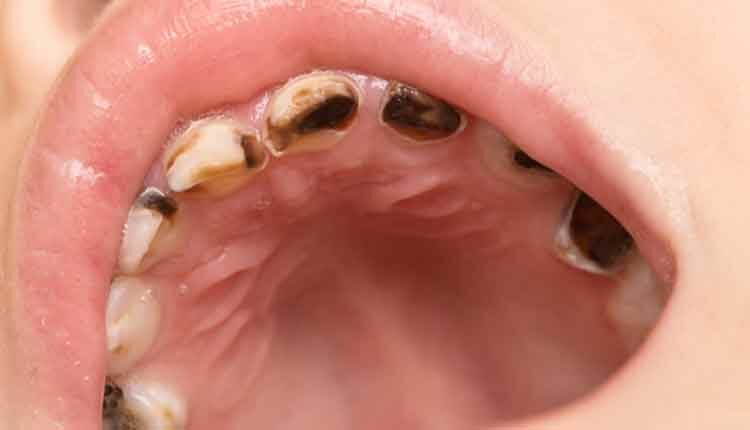
Early childhood caries (ECC) is a potentially devastating form of tooth decay in young children. The American Dental Association regards it as a significant public health problem, defining it as “… the presence of one or more decayed (noncavitated or cavitated lesions), missing (due to caries) or filled tooth surfaces in any primary tooth in a preschool-age child between birth and 71 months of age.”1
Not only can EEC wreak havoc with oral health, it can also significantly impact overall health and development, even interfering with a child’s ability to eat and speak.
Not long ago, researchers at the University of Pennsylvania School of Dental Medicine studied the interaction between the bacteria Streptococcus mutans and the fungus Candida albicans and its role in ECC.2 The highly acidic biofilm that results from this cross-kingdom association is particularly sticky and difficult to remove from tooth surfaces. The process is mediated through an enzyme, glucosyltransferases (GtfB), secreted by S. mutans, which binds to mannans, or molecules on C. albicans cell walls.
Mannans bind so tightly to Gftb, that a glue-like polymer forms in the presence of sugars. But treatment with antimicrobial agents alone isn’t always enough to degrade this type of biofilm. In addition, such treatment may also kill beneficial bacteria.
ENZYMATIC APPROACH
Building on their earlier research, the investigators conducted a new study to uncover a strategy for disrupting this tenacious form of plaque, investigating the efficacy of using enzymes to break up the GtfB-mannan interactions.3
The study involved the use of three mannan-degrading enzymes: one endoenzyme and two exoenzymes. These enzymes, which operate inside and outside cell walls, are known to be highly effective in reducing biofilm biomass without killing microorganisms. They also reduce the production of an acidic pH environment—the sweet spot for cariogenesis.
The enzymes were applied to biofilm growing on hydroxyapatite discs in human saliva medium and left for 5 minutes. The researchers found that, particularly in the case of the endoenzyme, overall biofilm volume, thickness, and bacteria-yeast interactions were reduced to the point that biofilm was more easily removed.
REDUCED DEMINERALIZATION
Research analysis showed a significant decrease in binding forces of GtfB to C. albicans following enzyme treatment. Not only did this disrupt biofilm mechanical stability but also significantly reduced demineralization without harming gingival keratinocytes.
While the enzymes were left for 5 minutes in the study, the researchers hope to see activity in the 2 minutes recommended for toothbrushing. Hwang says a nonalcohol-based mouthrinse with these enzymes added could potentially be used as a preventive measure against ECC.3
The researchers believe that targeting the bacterial-yeast binding interactions at the cellular level could yield significant nonbiocidal therapeutic intervention against pathogenic bacterial-fungal biofilms.
Until the advent of such a formulation, there are several nonalcohol-based mouthrinses available. Far from only promoting fresh breath, some rinses contain agents such as chlorhexidine gluconate to control plaque and gingivitis. Others are designed to treat conditions such as dry mouth and hypersensitivity.
REFERENCES
- American Dental Association. Statement on Early Childhood Caries. Available at: https://www.ada.org/en/about-the-ada/ada-positions-policies-and-statements/statement-on-early-childhood-caries.
- Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, Koo H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017;13:e1006407.
- Kim HE, Dhall A, Liu Y, Bawazir M, Koo H, Hwang G. Intervening in symbiotic cross-kingdom biofilm interactions: a binding mechanism-based nonmicrobicidal approach. mBio. 2021;12(3):e00651–00721.

