Probiotics and Periodontal Health
Emerging evidence highlights the potential of probiotics as a noninvasive adjunct to conventional periodontal treatments with promising benefits for oral and systemic health.
This course was published in the September/October 2025 issue and expires September 2028. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 490
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the role of the oral microbiome in maintaining health and the consequences of dysbiosis in periodontal diseases.
- Explain the therapeutic potential and mechanisms of action of specific probiotic strains in managing periodontal conditions.
- Analyze different delivery systems for administering probiotics in periodontal therapy and determine their potential effectiveness in clinical settings.
- Discuss appropriate probiotic delivery methods and safety considerations when recommending probiotics for periodontal care.
Periodontal diseases are multifactorial and chronic inflammatory conditions that impact the oral and systemic health of nearly half of the United States. More than 700 bacteria are present in the oral environment;1 however, not all bacteria are related to disease.
The oral microbiome is composed of bacteria, viruses, fungi, and protozoa. It is the second largest microbial community after the gut and is an ideal habitat for bacteria to flourish. The oral microbiome exists primarily as a dental biofilm and plays a vital role in protecting the mouth by maintaining a symbiotic relationship with the host. This balanced microbial community supports immune functions, inhibits the growth of harmful pathogens, and prevents pathogenic bacteria from proliferating in the oral environment.1
The oral microbiome can change with diet as well as oral pH.1 The problem arises when commensal bacteria are replaced with disease-related bacteria. With dysbiosis in the microbiome, a host response occurs, and the patient is susceptible to inflammatory diseases, such as periodontitis, as well as systemic diseases including diabetes, cardiovascular disease, and autoimmune diseases.2 Preventing dysbiosis is key to maintaining health.
The dental community has been searching for treatments that will supplement conventional methods of treating periodontal diseases. Nonsurgical periodontal therapy is hindered by limitations including root concavities, reliance on clinician skill, and scaling without direct vision. Research suggests scaling does not remove all the pathogenic bacteria, leading to reinfection of the pocket and the risk of antibiotic resistance discourages the use of this therapy.3 Surgical intervention is often the only option.3
A Noninvasive Approach
Probiotics are one of the most recent therapies implemented to protect the oral environment. Live microorganisms that provide health benefits to the host, probiotics were originally used to treat intestinal issues and improve lactose intolerance. More recently, probiotics have been utilized to prevent and treat oral diseases, such as caries, periodontal diseases, oral candidiasis, and oral malodor.4 Probiotics offer several health benefits, including the production of lactic acid, which has antibacterial properties, and hydrogen peroxide, which acts as an antiseptic with antiviral and antifungal effects — together helping to suppress harmful microorganisms.5
Periodontal diseases may benefit from the therapeutic potential of probiotics. They have demonstrated reduction in plaque biofilm levels and bleeding on probing, in addition to improving clinical attachment levels (CAL).4 As traditional methods of treating periodontal diseases have not completely blocked progression, other treatments, such as probiotics, should be investigated.6 Additionally, the use of probiotics positively impacts alveolar bone levels. Studies suggest that less bone loss occurs with the use of probiotics than without probiotics. Also used in conjunction with nonsurgical periodontal therapy, probiotics help reduce attachment loss.7
Probiotics inhibit the growth of periodontal pathogens and modulate the host immune response in order to reduce inflammation and prevent tissue destruction. Probiotics help regulate periodontal pathogens by inhibiting their growth, attachment, and biofilm formation; suppressing the activity of harmful bacterial genes; and restoring balance between aerobic and anaerobic bacteria in the periodontal environment.3 Also, probiotics can affect the host response by reducing pro-inflammatory factors and increasing anti-inflammatory factors, controlling the number of inflammatory cells, boosting the production of antimicrobial peptides, and counteracting pro-inflammatory factors triggered by periodontal bacteria.
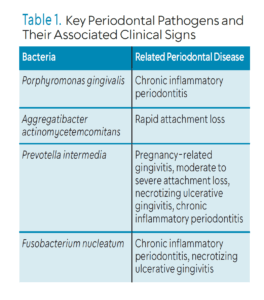
Most of the research on probiotics has focused on Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, and Fusobacterium nucleatum, which have all been implicated in periodontal diseases (Table 1).3,6 During the active phase of periodontal disease, reactive oxygen species (ROS) is overproduced. This eventually leads to tissue damage and greater loss of attachment. Probiotics activate antioxidant pathways, boost the expression of various antioxidant enzymes, and suppress inflammatory processes that contribute to excessive ROS production. This helps reduce oxidative damage and inflammation in periodontal tissues.3
![]() Specific Probiotic Strains
Specific Probiotic Strains
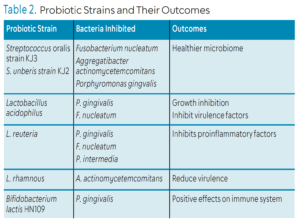
The efficacy of probiotics is important when deciding if they should be recommended to patients (Table 2).8 To be effective in influencing the disease process, probiotics must provide the following:8
- Attach to the tooth surface or mucosa so they are not easily washed away
- Generate antimicrobial compounds with the ability to kill pathogens
- Reduce inflammatory activities and change the microbial environment in the oral cavity
- Be clinically validated and have a demonstrated health effect
Streptococcus is a bacterium that is potentially effective as a probiotic because it is commensal in the microbiome and supports tooth and gingiva development. Streptococcus spp. have a strong adhesion to the oral cavity, so they are not easily displaced by eating or saliva flow.9 Streptococcus produce metabolic byproducts that suppress the growth of harmful pathogens. The mechanism of action is through production of hydrogen peroxide that inhibits bacterial growth. The two strains that most benefit the oral cavity are S. oralis strain KJ3 and S. uberis strain KJ2.11 They inhibit F. nucleatum, A. actinomycetemcomitans, and P. gingivalis. Colonization of S. oralis and S. uberis on the salivary pellicle leads to a healthier microbiome.11
Lactobacillus is a Gram-positive facultive anaerobic or obligate anaerobic bacterium that can be found in the digestive system, urinary system, and reproductive system. L. acidophilus inhibits the growth of P. gingivalis and reduces the virulence factors of F. nucleatum. L. reuteri inhibits the growth of P. gingivalis, F. nucleatum, and P. intermedia. They compete with these periodontal pathogens for adhesion sites. L. reuteri also inhibits proinflammatory factors. L. rhamnous interferes with the host response by reducing biofilm biomass and blocking the growth of P. gingivalis and A. actinomycetemcomitans. L. rhamnous also reduces virulence of A. actinomycetemcomitans.4
Bifidobacterium is found naturally in the gut and oral cavity. B. lactis HN019 has demonstrated positive effects on the immune system. It can reduce inflammatory cytokines such as IL-1ß levels and ratio of RANKL-OPG as well as regulate expression of TNF-a and IL-6. The lactic acid found within Bifidobacterium can disintegrate outer membrane of Gram-negative bacteria. Bifidobacterium can also reduce adhesion of P. gingivalis.10
Delivery of Probiotics
A probiotic’s delivery method may influence its duration of activity and overall effectiveness. Probiotics have been dispensed through lozenges, tablets, sachets, capsules, toothpastes, mouthrinse, and gel drops. There is a direct correlation between the length of use, dose of probiotic, and reduction in clinical signs of inflammation.4
Currently, only a few probiotic products target the oral environment specifically. Most products contain a combination of different probiotic strains.11 When choosing a probiotic to enhance oral health, the following should be considered: probiotic strain, adherence ability, and effectiveness in inhibiting pathogens.11
Lozenges and tablets are frequently used due to the amount of time they are in the oral environment. Directions include suck, chew, or dissolve in the oral cavity. Patients with dysphagia or difficulty chewing should not be given tablets or lozenges. Lozenges with L. reuteri, S. salivarius, and L. paracasei have shown a reduction in plaque, bleeding on probing, and decrease of A. actinomycetemcomitans, P. gingivalis, P. intermedia, and F. nucleatum.11 L. reuteri in lozenge form also reduced obligate anaerobes implicated in periodontitis.11
Probiotic mouthrinses are less common than other delivery methods but show promise for future use. A rinse containing multiple probiotic strains, such as L. acidophilus, L. rhamnosus, B. longum, and S. boulardii, was shown to reduce gingival inflammation, decrease pocket depth, and improve clinical attachment in participants who used it twice daily for 30 days.13 Given concerns that chlorhexidine may inhibit fibroblast production, a probiotic alternative that reduces clinical signs of disease could be a valuable option.12 However, one drawback of mouthrinses is the difficulty in delivering an accurate, consistent dose.11
Toothpaste may serve as a delivery system for probiotics. A toothpaste containing L. paracasei demonstrated a positive effect on bleeding on probing, gingival inflammation, and plaque reduction.15 One study demonstrated that probiotics in toothpaste inhibited bacteria better than in mouthrinse. It was suspected the combination of the toothbrush and toothpaste ensured the probiotic reached down in the pocket easier than the mouthrinse.6
Chewing gum has also been utilized to deliver probiotics. Shirbhate et al8 found that L. reuteri has the potential to decrease clinical markers of inflammation after 2 weeks of use. Oral strips are another promising delivery system as they exhibit antioxidant properties, maintain stable shelf life, stick to the oral mucosa, can be used by those with dysphagia, and are site specific.11
Eating fermented foods is another way to increase probiotic exposure. The fermenting process produces healthy bacteria and provides gut health benefits. Kimchi, tempeh, miso, sauerkraut, kefir, yogurt, and cheese have gone through this preservation process. In 1965, the term probiotics was used to describe these beneficial bacteria in the fermentation process.5 Probiotics in food and beverages can inhibit oral pathogens.11 Data from the National Health and Nutrition Examination Survey 2009-2014 show that those who consumed probiotics had a reduced risk of developing periodontitis.16 This information can be utilized when educating patients on nutrition.
Safety and Regulation
The regulation of probiotics by the US Food and Drug Administration (FDA) is complex. The FDA oversees products marketed as food ingredients or medications; however, many probiotics are sold as dietary supplements, which are not subject to FDA regulation. If a probiotic is intended to treat a disease, it must undergo clinical trials to demonstrate safety and effectiveness to receive FDA approval.17
When incorporating a probiotic into a treatment plan, safety should be considered to avoid antibiotic resistance. Probiotic strains must undergo a thorough genetic evaluation to confirm the absence of acquired or transferable antibiotic resistance genes and to assess their genomic stability. Probiotics need to also be considered for bile salt hydrolase activity (BSH), which puts patients at risk for gallstone formation. Lactobacillus and Bifidobacterium have BSH activity; therefore, these strains should not be used in patients at risk for gallstones.18,19
Emerging evidence shows that probiotics may act as opportunistic pathogens for individuals who are immune compromised, stressed, older, or newborn. Caution should be used for patients with have life-threatening pneumonia, endocarditis, and sepsis. Long-term studies are warranted for evaluating continued use of probiotics. If the probiotic is classified as a dietary supplement, there is no consistent label on safety. Patients with health risks may want to avoid taking dietary supplements.20
Facilities producing probiotics need to follow the good manufacturing practices that are recognized globally. This will assure the product can be traced back to manufacturer and the facility can be tested if a problem is found. It also assures proper monitoring and controls, and appropriate risks are identified in the process. Potential allergens need to be disclosed on the packaging. The list of ingredients should be reviewed before recommending a probiotic.21
Conclusion
Periodontal diseases pose many challenges for dental hygienists and the use of therapies beyond the traditional methods may be needed. Probiotics may be beneficial when treating gingivitis and periodontitis as an adjunct to nonsurgical periodontal therapy. Probiotics may help reduce plaque biofilm and bleeding on probing and improve CAL.4 The key is to choose the right probiotic for the patient and the right delivery system. Caution does need to be taken if the patient is immunocompromised or presents with serious health risks.
References
1. Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23:122-128.
2. Georges F, Do NT, Seleem D. Orals dysbiosis and systemic diseases. Front Dent Med. 2022:3.
3. Baddouri L, Hannig M. Probiotics as an adjunctive therapy in periodontitis treatment—reality or illusion—a clinical perspective. NPJ Biofilms Microbiomes. 2024;10:148.
4. Hardan L, Bourg, R, Cuevas-Suarez C, et al. The Use of probiotics as adjuvant therapy of periodontal treatment: a systematic review and meta-analysis of clinical trials. Pharmaceutics. 2022;14(5):1017
5. Gatej S, Gully N, Gibson R, Bartold M. Probiotics and periodontitis a literature review. J Periodontol. 2017;19:42-50.
6. Zhang Y, Ding Y, Guo Q. Probiotic species in the management of periodontal diseases: an overview. Front Cell Infect Microbiol. 2022;12:806463.
7. Nguyen T, Brody H., Radaic A, Kapila Y. Probiotics for periodontal health current molecular findings. Periodontol 2000. 2021;87:254-267.
8. Shirbhate U, Bajaj P, Chandak M, et al. Clinical implications of probiotics in oral and periodontal health: a comprehensive review. Cureus. 2023.15:e51177.
9. Beattie RE Probiotics for oral health: a critical evaluation of bacterial strains. Front Microbiol. 2024:15:1430810.
10. Deandra F, Ketherin K, Rachmasari R, Sulijaya B, Takahashi N. Probiotics and metabolites regulate the oral and gut microbiome compostition as host modulation agents in periodontitis: a narrative review. Heliyon. 2023;3;9:e13475.
11. How YH, Yeo SK. Oral probiotic and its delivery carriers to improve oral health: a review. Microbiology (Reading). 2021;167:001076.
12. Ranjith A, Nazimudeen N, Baiju K. Probiotic mouthwash as an adjunct to mechanical therapy in treatment of Stage II periodontitis : randomized clinical trial. Int J Dent Hyg. 2022;20:415-421.
13. Mariotti AJ, Rumpf D.A. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J Periodontol. 1999; 70:1443–1448.
14. Soares H, Kaio F, Parsa MS, et al. Efficacy of probiotics compared to chlorhexidine mouthwash in improving periodontal status: a systematic review and meta-analysis. Int J Dent. 2023:4013004.
15. Li X, Zhao Z, Guo S, et al. Effects of toothpaste containing inactivated Lacticaseibacillus paracasei Probio-01 on plaque-induced gingivitis and dental plaque microbiota. Microbial Pathogenesis. 2024;192:2024.
16. Ren Z, Xue Y, Zhang H, et al. Association between probiotic consumption and periodontitis: evidence from NHANES 2009–2014. J Clin Periodontol. 2023;50:1476–1486.
17. National Center for Complementary and Integrative Health. Probiotics: Usefulness and Safety. Available at nccih.nih.gov/health/probiotics-usefulness-and-safety. Accessed August 7, 2025.
18. Begley M, Hill C, Gahan CGM. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72:1729-1738.
19. Park J, Lee J, Kim Y, Kim B, Kim B, Choi S. Biosafety characteristics antibacterial activity of probiotic strains against Streptococcus mutans, Aggregatibacter actinomycetemcomitans, and Porphyromaonas gingivalis. Ann Microbiol. 2025;75:2.
20. Merenstein D, Pot B, Leyer G, et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. 2023;1:1.
21. Amy L, Roe, Boyte ME, Elkins CA, et al. Considerations for determining safety of probiotics: A USP perspective. Regul Toxicol Pharmacol. 2022:136:105266.
From Dimensions of Dental Hygiene. September/October 2025; 23(5):36-39.