Get Infection Control Right Every Time With Spore Tests and Sterilizers
Spore testing helps dental teams verify sterilizer performance, protect patients, and stay compliant with infection control standards.
Sterilization monitoring is a cornerstone of infection control and prevention in dental settings. Spore testing, also known as biological monitoring, is considered the gold standard for verifying the efficacy of sterilization processes. Oral health professionals must routinely verify that their sterilizers are working properly, including performing spore tests at the frequency recommended by the United States Centers for Disease Control and Prevention (CDC) and required by the Occupational Safety and Health Administration (OSHA) Bloodborne Pathogens Standard. The two most common types of spore testing used in dental practices are in-office incubation and mail-in.
Regulatory Background
According to the CDC’s Guidelines for Infection Control in Dental Health-Care Settings, sterilization procedures should be monitored using biological indicators (spore tests) at least weekly and with every load that contains implantable devices, such as titanium or ceramic zirconia implants.1 OSHA reinforces this recommendation under the Bloodborne Pathogens Standard (29 CFR 1910.1030), which mandates that employers ensure proper functioning of sterilization equipment as part of their exposure control plan.2
Importance of Sterilization in Dentistry
Sterilization of dental instruments is a critical component of infection prevention in dental practices. Instruments used during patient care come into contact with blood, saliva, and other potentially infectious materials, posing a risk of cross-contamination if not properly sterilized.1 Failure to adequately sterilize instruments can result in the transmission of pathogens.3
The Spaulding classification system is a widely accepted framework for determining the level of disinfection or sterilization required for medical and dental instruments. The Spaulding classification categorizes instruments into three levels based on the risk of infection associated with their use: critical, semicritical, and noncritical.1
Critical items are instruments that penetrate soft tissue or bone (eg, scalers, surgical instruments). These must be heat sterilized after each use. Semi-critical items are instruments that contact mucous membranes but do not penetrate soft tissue (eg, mouth mirrors, reusable impression trays). These should also be heat sterilized after each use. Noncritical items are instruments or devices that only contact intact skin (eg, blood pressure cuffs, X-ray tube head).1 These require intermediate or low-level disinfection depending on the level of contamination.
Sterilization protects both patients and oral health professionals by ensuring that instruments are free from all forms of microbial life, including spores. This process must be consistent, validated, and documented as part of a comprehensive infection control program.1,2 Effective sterilization reassures patients about the safety of dental care and fulfills the legal and ethical responsibility of the provider.
Sterilization Monitoring
The CDC provides clear guidance on sterilization monitoring in dental practices to ensure patient safety and prevent disease transmission. Three types of monitoring are recommended by the CDC and required by the OSHA Bloodborne Pathogens Standard as a comprehensive quality assurance system for sterilization procedures.1,2 Monitoring includes mechanical, chemical, and biological monitoring.
Mechanical monitoring includes observing or visually verifying the gauges on the sterilizer including time, temperature, and pressure readings, which should be conducted during each sterilization cycle.1 Some new sterilizers include technology that prints out mechanical monitoring results on a tape roll or through removable USB devices, wireless internet connection, or cloud storage. New technology can eliminate the need for visual monitoring, improving staff efficiency.
Chemical monitoring includes the use of chemical indicators (CI), which should be used with every package processed, including both external and internal indicators, to visually verify that sterilization conditions were met. CIs are designed to react to one or more parameters of the sterilization process, typically time, temperature, and presence of steam or gas.1,4,5 These indicators provide immediate visual confirmation that the sterilization cycle has reached the required conditions for efficacy, although they do not prove microbial kill like a biological spore test.
 CIs are located on sterilization pouches, which turn color when the parameters are reached or as indicator strips, known as integrators, are placed inside of a cassette, pouch, or bag. CIs should be used in every package, tray, or load to provide immediate feedback on whether sterilization parameters were met. When used consistently with biological monitoring, CIs enhance patient safety and support compliance with infection prevention protocols.
CIs are located on sterilization pouches, which turn color when the parameters are reached or as indicator strips, known as integrators, are placed inside of a cassette, pouch, or bag. CIs should be used in every package, tray, or load to provide immediate feedback on whether sterilization parameters were met. When used consistently with biological monitoring, CIs enhance patient safety and support compliance with infection prevention protocols.
Biological monitoring should be performed at least weekly (as a minimum) and whenever a sterilizer is newly installed, repaired, or relocated.1,5 The CDC also emphasizes the importance of documenting the results of each spore test, maintaining records in compliance with OSHA, and taking immediate corrective action in the event of a failed test result. Results of all monitoring should be recorded and documented for compliance.1,2
While biological indicators (BIs), or spore tests, are the most reliable method for monitoring sterilization, CIs also play a vital role in daily sterilizer performance checks. Used alongside BIs, CIs offer additional layers of assurance and are essential for maintaining quality control in sterilization procedures. Table 1 outlines the six types of CIs.
The CDC also recommends that dental practices develop and follow written policies and procedures for recording and documenting results of sterilization monitoring and for sterilization processes, including response protocols for positive BI tests (failed spore tests).1,4
Instruments should not be used until a passing spore test confirms sterilizer performance.4,6,7 Failure to remove a malfunctioning sterilizer, or to recall and reprocess potentially contaminated instruments, may result in significant health risks and regulatory penalties.4
Types of Sterilizers Used in Dentistry
Several types of sterilizers exist, each designed to meet specific needs based on instrument load, material type, and workflow. Oral health professionals must understand the instructions for use (IFU) for each sterilizer machine used in the practice setting, as each machine operates differently. Consulting the manufacturer’s IFU for loading and operating instructions vs relying on previous experience or legacy errors is prudent. The manufacturer’s IFU will also outline the recommended types of chemical and biological monitoring products to use, guiding the user on compatibility features. Therefore, understanding the sterilizer specifics will guide the user on best practices with chemical and biological monitoring in their setting. Common types of sterilizers are:4
- Steam Sterilizers (Autoclaves)
- Use pressurized steam to achieve sterilization
- Fast, effective, and compatible with most dental instruments; common in dentistry
- Available in gravity displacement and pre-vacuum (high-speed) models
- Dry Heat Sterilizers
- Use high temperatures without moisture to sterilize instruments
- Suitable for instruments that may corrode or dull with steam
- Require longer cycle times than steam sterilizers
- Chemical Vapor Sterilizers
- Use a mixture of chemicals heated under pressure to sterilize
- Less corrosive than steam; appropriate for heat- and moisture-sensitive instruments
- Require adequate ventilation due to chemical emissions
- Unsaturated Chemical Vapor Sterilizers and Gas Plasma
- Used less frequently in dentistry
- Effective for delicate, heat-sensitive items
Sterilizer choice depends on factors, such as instrument compatibility, sterilization time, environmental controls, and cost. Regardless of type, all sterilizers must be maintained, calibrated, and monitored using mechanical, chemical, and BIs to ensure optimal performance.1,2
Biological monitoring evaluates whether a sterilization cycle effectively kills highly resistant microorganisms, typically bacterial endospores from Geobacillus stearothermophilus or Bacillus atrophaeus.1 This form of testing can detect failures in the sterilization process that may not be identified by mechanical means or CIs alone. Two methods are commonly used in dental offices: in-office testing kits and mail-in biological monitoring services. Table 2 outlines the process for conducting both methods of spore testing.
Spore Testing
In-office spore testing kits allow dental practices to incubate and read BIs on-site. These kits typically consist of spore strips or self-contained vials and an incubator. The turnaround time for results is faster, typically within 24 to 48 hours, allowing immediate identification of sterilization failures.4 Newly developed ultra-rapid incubators can provide results within 20 to 60 minutes. Ultra-rapid BI testing uses advanced detection methods, such as bioluminescence, to provide rapid results vs slower culture methods.8
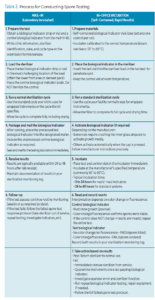 The advantages of in-office spore testing include immediate feedback and rapid results. The user determines when testing and incubation occurs, making it convenient and controllable. The initial kit includes a one-time incubator purchase. Spore testing vials are the only supply that is needed after the initial purchase. Long-term, this method can be cost effective.
The advantages of in-office spore testing include immediate feedback and rapid results. The user determines when testing and incubation occurs, making it convenient and controllable. The initial kit includes a one-time incubator purchase. Spore testing vials are the only supply that is needed after the initial purchase. Long-term, this method can be cost effective.
Some of the disadvantages of in-office spore testing include time and training. Staff training is required initially to understand the process of adherence to incubation protocols. Therefore, there is a risk of human error during processing or interpretation. It is crucial to maintain the incubator and monitor the temperature accurately.
Mail-in testing involves sending BIs to a laboratory for incubation and result reporting. A control and an exposed spore strip are enclosed in a special envelope provided by a vendor and mailed in. The process is generally outsourced to third-party vendors who provide detailed reporting and quality assurance services.
The advantages of mail-in spore testing include low cost and ease of use. Mail-in envelopes, including the control strip and the spore strip, are provided and are relatively inexpensive. The user places the strip into the sterilizer in a challenging area, and it is run through a regular sterilization cycle.
Challenging areas include the center of a gravity displacement steam sterilizer, or near the door of a pre-vacuum steam sterilizer. Once completed, the strip is placed back into the envelope, the envelope is labeled and mailed to the vendor for laboratory incubation. This method reduces in-office workload and eliminates the need for an incubator. The spore testing is performed by trained laboratory personnel upon arrival. Results are documented and reported back to the office after 48 hours of incubation.
Some of the disadvantages of mail-in spore testing include delayed results and the risk of getting lost in the mail. The slower mail-in method might delay results for up to 7 days. The risk of an unknown delayed spore test failure could result in contaminated instruments being used, forcing an instrument recall or worst-case scenario of contacting patients.
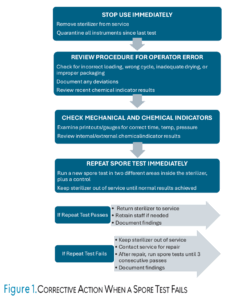
Regardless of the testing method used, documentation and recordkeeping are essential for OSHA compliance.2 Sterilizer monitoring records must be maintained for 1 to 3 years depending on state regulations and should include date, sterilizer used, type of test, results, and actions taken if results indicate failure.2 The CDC recommends maintaining records for 3 years.1 Dental practices should develop written policies that outline procedures for biological monitoring and corrective actions in the event of sterilization failure. Figure 1 outlines the steps and corrective action needed when a spore test fails.
 The CDC does not specify a preferred method (in-office vs mail-in) but emphasizes the need for regular weekly testing and proper documentation.1 Practices must also verify that spore test strains, incubation methods, and result interpretations follow manufacturer instructions and scientific standards.
The CDC does not specify a preferred method (in-office vs mail-in) but emphasizes the need for regular weekly testing and proper documentation.1 Practices must also verify that spore test strains, incubation methods, and result interpretations follow manufacturer instructions and scientific standards.
Best Practices for Spore Testing
Several best practices are needed to ensure compliance with biological monitoring. First, a trained staff member or infection control coordinator should be designated to oversee sterilization monitoring. Having one or two staff members responsible for this important task eliminates human error and enhances correct safety procedures.
Biological monitoring should be performed at least weekly (at a minimum) and whenever a sterilizer is newly installed, repaired, or relocated.1,4 Second, a method for biological monitoring should be chosen that aligns with the practice’s size, volume, and workflow. High-volume practices with rapid instrument turnover might consider in-office methods for quicker results. Third, perform and log results at least weekly, or more often depending on the practice volume, as well as after sterilizer repairs or failures. It is vital to keep backup indicators and supplies available. Finally, immediately investigate and document any positive test results (failed spore tests) and take corrective action. Failed spore tests happen and are often a result of human error such as overloading the sterilizer.
Conclusion
Sterilization is non-negotiable in dental settings and spore testing is an essential component of verifying sterilizer effectiveness. Both in-office incubation and mail-in methods have merits and limitations. Dental practices must choose the most appropriate option based on workflow, resources, and compliance needs. Consistent monitoring, accurate documentation, and staff training are crucial.
References
- United States Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care Settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1–61.
- Occupational Safety and Health Administration. 29 CFR 1910.1030 – Bloodborne Pathogens. Available at osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030. Accessed December 16, 2025.
- United States Centers for Disease Control and Prevention. COVID-19 Guidance for Dental Settings. Available at cdc_88256_DS1.pdf. Accessed December 16, 2025.
- United States Centers for Disease Control and Prevention. Best Practices for Sterilization Monitoring in Dental Settings. Available at cdc.gov/dental-infection-control/hcp/dental-ipc-faqs/sterilization-monitoring.html. Accessed December 16, 2025.
- Association for the Advancement of Medical Instrumentation (AAMI). Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities. Available at https://array.aami.org/doi/book/10.2345/9781570208027. Accessed December 16, 2025.
- Sasaki J, Imazato S. Autoclave sterilization of dental handpieces: A literature review. J Prosth Res. 2020;3: 239-242.
- Patiño-Marín N, Villa García L, Aguirre López E, et al. Sterilization and disinfection: ensuring infection control in dental practices. Cureus. 2025;17:e79041.
- Rams TE, Sautter JD, Lee AH, van Winkelhoff AJ. Evaluation of a rapid biological spore test for dental instrument sterilization. J Contemp Dent Pract. 2022;23:279-283.
From Dimensions of Dental Hygiene. January/February 2026; 24(1):9-12

