Buffered Articaine Provides the Next Leap in Dental Pain Management
Combining articaine’s superior diffusion with the rapid comfort and onset benefits of buffering technology promises to elevate patient care.
It may appear that the evolution of dental local anesthesia has been slow to progress. At a minimum, profound local anesthesia requires an understanding of anatomy and a familiarity with drug delivery devices as well as safe and effective drugs. Anatomical features have not changed. Since the original Cooke Waite syringe, delivery devices have undergone only minimal changes; however, pharmacology has made significant advances. Current evidence and anticipated new technologies suggest that progress is moving forward.
Reflecting on current practice, articaine and chairside buffering are prime examples of dentistry’s response to earlier limitations. Clinicians are much better equipped today to meet pain management challenges.1-3
Lidocaine
Lidocaine has provided reliably effective and durable pain management since the late 1940s and is frequently referred to as the “gold standard” of dental local anesthetics (LAs). Despite the subsequent introduction of articaine in 2000, lidocaine has maintained its dominance in the United States marketplace.3 However, Business Research Insights projects that by 2033, articaine’s global market share will be more than three times that of lidocaine.4
Articaine and Buffered Articaine
Combining the pharmacological advantages of both articaine and buffering, regardless of the injection technique, can significantly improve injection comfort and pain management during procedures.1,2 In addition to the key benefits of buffering, articaine also offers enhanced diffusion, effective 1:200,000 epinephrine concentrations, a comparatively short half-life, and unique metabolic pathways that largely avoid liver metabolism, reducing risks among individuals with compromised liver function.1,3
A study comparing the diffusibility of 4% articaine and 2% lidocaine in palatal tissues after buccal infiltration found a significant difference, with 4% articaine reaching markedly higher concentrations in the palatal mucosa.5 Rathi et al6 compared 2% lidocaine, 1:80,000 epinephrine vs 4% articaine, 1:100,000 epinephrine in buccal infiltration for extraction of maxillary and mandibular primary molars in 7- to 12-year-old patients. None of the patients who received 4% articaine needed palatal or lingual injections while all patients who received 2% lidocaine needed palatal or lingual injections. While none of the drugs in these studies were buffered, articaine in this and other studies has demonstrated distinct benefits over lidocaine.7,8
Studies comparing nonbuffered to buffered articaine injections have noted significant improvements with the use of buffering, including comfort, rapid onset, effectiveness, and duration.1,2,9-14 A 2022 study comparing these factors found buffered articaine to be superior to a nonbuffered control.13 More recently, Staedt et al9 reported superior anesthetic efficacy, reduced pain and onset, and increased duration when comparing buffered to nonbuffered articaine. In summary, combining the pharmacological advantages of both articaine and buffering, regardless of the injection technique and clinician skill, can significantly improve outcomes. Buffered articaine is effective in both 1:100,000 and 1:200,000 epinephrine concentrations.
Science of Buffering
Drug solutions are acidified during manufacturing to enhance solubility and increase stability in cartridges.3 In addition, solutions with vasoconstrictors are more acidic because they include sulfite antioxidants to improve vasoconstrictor shelf-life, which further lowers pH levels (Figure 1).3,14 Throughout the useful shelf-life of LA drugs with vasoconstrictors, these preservatives continue to increase acidity, which can delay onset and increase burning and stinging sensations.
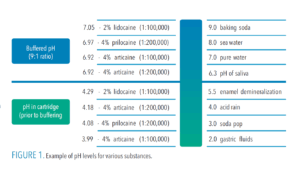 As acidity increases, the percentage of charged molecules (RNH+) initially deposited into tissues is increased exponentially. An LA drug with a pH of 6.4, for example, is 10x more acidic than physiological pH (7.4), a pH of 5.4 is 100x more acidic than 6.4, and a pH of 4.4 is 1,000x more acidic than 7.4.15
As acidity increases, the percentage of charged molecules (RNH+) initially deposited into tissues is increased exponentially. An LA drug with a pH of 6.4, for example, is 10x more acidic than physiological pH (7.4), a pH of 5.4 is 100x more acidic than 6.4, and a pH of 4.4 is 1,000x more acidic than 7.4.15
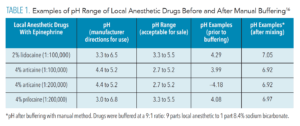
Physiologic or innate carbonate buffering in human tissues quickly raises the pH of LA drugs after injection (Table 1).16 Cartridges of 2% lidocaine with 1:100,000 epinephrine may range from as low as pH 2.9 to 4.4.17 In this range, the initial percentage of uncharged molecules (RN) that are available to cross nerve membranes is minimal, significantly less than in extracellular tissues, which are slightly alkaline at a typical pH range of 7.2-7.4+.18 According to Jain et al,13 at a pH of 3.5, only 0.004% of 2% lidocaine molecules are initially available to cross nerve membranes. When LA drugs are buffered to a near neutral pH, approximately half of the (RN) molecules are available to cross nerve membranes.
Compared to physiological buffering, pharmacological buffering has significant benefits, including a much more rapid increase in pH. As early as 1972, Catchlove19 provided insight into the theory of pharmacological buffering of LA agents. He described mechanisms by which CO2, that is generated when actively buffering LA drugs, is thought to enhance comfort, onset, efficacy, and duration. It is known to have a rapid direct depressant effect on the nerve membrane (comfort, fast onset, and increased duration), it decreases axoplasmic pH by forming carbonic acid, which concentrates RNH+ ions (increased effectiveness). And it results in an additional trapped surplus of RNH+ in the axoplasm (further enhanced ion channel gating, ie, effectiveness).19 These rapid actions of CO2 appear to account for pharmacological buffering’s overall enhanced benefits.8,9,12,19
For optimal results, the buffering agent should be mixed with the LA drug as near as possible to the time of injection. A 2024 study of nonbuffered vs buffered 4% articaine, 1:200,000 epinephrine during maxillary third molar extractions found that buffered articaine provided superior efficacy, enhanced patient comfort, accelerated onset, and prolonged duration of action.9
In medicine, buffering of LAs began 60 years ago.14 Due to cartridge design, it has been more difficult to add buffering agents in dentistry. This and cost may have contributed to hesitance to adopt buffering or to limit its use to patients who are difficult to anesthetize.
New Buffering Technology
New technology features the ability to add single unit doses of sodium bicarbonate buffering to prepackaged dental cartridges, which can reduce waste that may result from the expiration of unused buffering agent in devices that contain multiple doses.
Preloaded cartridges are anticipated to be available in the next few years. These will fit into any dental syringe or device that can accommodate cartridges. The unique design will likely include separate chambers for buffering agent and LA drug solution. When loaded into a syringe, buffering agent in one chamber will rapidly mix with the LA drug. This will eliminate the need for any secondary mixing techniques.
Conclusion
According to Rubin,20 hesitance to adopt well-founded evidence despite credible research can delay its integration into practice for up to 17 years. This could easily span a large portion of a clinician’s career during which time patients do not benefit.
While many clinicians now include buffering in their pain management routines, others remain hesitant. Although there are current effective delivery methods (spanning more than 15 years), new technology has the potential to simplify chairside buffering even further, improving workflow.
References
- DiMarco AC, Bassett KB, Perspectives on buffered articaine in dentistry. Decisions in Dentistry. 2022;8(6):28-31.
- de Geus JL, Nogueira da Costa JK, Maran BM, Loguercio AD, Reis A. Different anesthetics on the efficacy of inferior alveolar nerve block in patients with irreversible pulpitis. J Am Dent Assoc. 2020;151:87–97.
- Bassett K, DiMarco A, Naughton D. Local Anesthesia for Dental Professionals. 2nd ed. Upper Saddle River, New Jersey: Pearson; 2015.
- Global Dental Anesthesia Industry Research Report by 2033. Available at businessresearchinsights.com/market-reports/dental-anesthesia-market-110296. Accessed August 11, 2025.
- Al-Mahalawy H, Abuohashish H, Chathoth S, Al-Masoud N, Al-Jandan B. Articaine versus lidocaine concentration in palatal tissues after supraperiosteal buccal infiltration anesthesia. J Oral Maxillofac Surg. 2018;76:315.e1–315.
- Rathi NV, Khati AA, Agrawal AG, Baliga M, Thosar NR, Deolia SG. Anesthetic efficacy of buccal infiltration articaine versus lidocaine for extraction of primary molar teeth. Anesth Prog. 2019; 66:3–7.
- Nagendrababu V, Duncan HF, Whitworth J, et al. Is articaine more effective than lidocaine in patients with irreversible pulpitis? An umbrella review. Int Endod J. 2020;53:200–213.
- Luo W, Zheng K, Kuang H, Li Z, Wang J, Mei J. The potential of articaine as new generation of local anesthesia in dental clinics: A review. Medicine (Baltimore). 2022;101(48):e32089.
- Staedt H, Palarie V, Heimes D, Ottl P, Fan S, Kämmerer PW. Buffered 4% articaine reduces pain and enhances anesthesia in maxillary third molar extractions: a randomized, double-blind split-mouth study. Biomedicines. 2024; 12:2691.
- Kattan S, Lee SM, Hersh E, Karabucak B. Do buffered local anesthetics provide more successful anesthesia than nonbuffered solutions in patients with pulpally involved teeth requiring dental therapy? A systematic review. J Am Dent Assoc. 2019;150:165-177.
- Donaldson M, Goodchild JH. Taking local anesthesia to the next level: Four strategies clinicians may consider. Compend Contin Educ Dent. 2022:43:20–24.
- Goodchild JH, Donaldson M. Comparing the pH change of local anesthetic solutions using two chairside buffering techniques. Compend Contin Educ Dent. 2016;37:e6–e12.
- Jain T, Jha R, Tiwari A, et al. A comparative study to evaluate the anesthetic efficacy of buffered versus non-buffered 2% lidocaine during inferior alveolar nerve block. Cureus. 2022;14:e31855.
- Davies R. Buffering the pain of local anaesthetics: A systematic review. Emerg Med (Fremantle). 2003;15:81-88.
- Hydrogen Ion Concentration Calculator. Available at omnicalculator.com/chemistry/hydrogen-ion-concentration. Accessed August 11, 2025.
- Goodchild JH, Donaldson M. Comparing the pH change of local anesthetic solutions using two chairside buffering techniques. Compend Contin Educ Dent. 2016;37:e6–e12.
- DiMarco A, Bassett KB, Implementing buffered articaine in dentistry. Dimensions of Dental Hygiene. 2022;20:40-43.
- Malamed S. Buffering local anesthetics in dentistry. Pulse. 2011;44:8–9.
- Catchlove R. The influence of CO2 and pH on local anesthetics in dentistry. Surv Amesthesiol. 1973; 17:184-185.
- Rubin R. It takes an average of 17 years for evidence to change practice—the burgeoning field of implementation science seeks to speed things up. J Am Med Assoc. 2023;329:1333–1336.
From Dimensions of Dental Hygiene. September/October 2025; 23(5):16-19.